810601P
Avanti
16:0-5 Doxyl PC
Avanti Research™ - A Croda Brand 810601P, powder
Synonyme(s) :
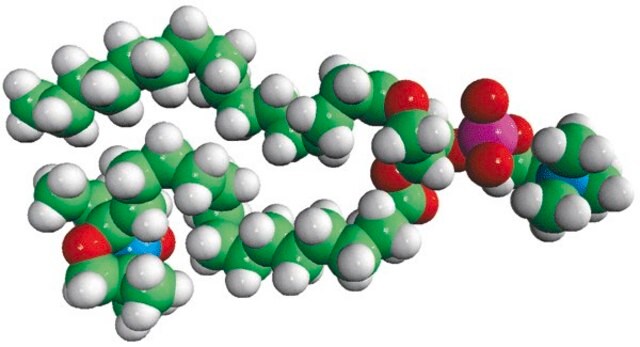
1-palmitoyl-2-stearoyl-(5-doxyl)-sn-glycero-3-phosphocholine
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C46H90N2O10P
Numéro CAS:
Poids moléculaire :
862.19
Code UNSPSC :
41141825
Nomenclature NACRES :
NA.25
Produits recommandés
Pureté
>99% (TLC)
Forme
powder
Conditionnement
pkg of 1 × 1 mg (810601P-1mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand 810601P
Type de lipide
ESR probes
phospholipids
Conditions d'expédition
dry ice
Température de stockage
−20°C
Description générale
Avanti′s nitroxide spin product listing is a group of compounds designed to act as membrane probes. A variety of positions down the hydrophobic chain are labeled with the nitroxide functional groups to allow probing the membrane at various depths. These compounds have been synthesized from 1-palmitoyl-2-hydroxy-sn-glycerol-3-phosphocholine with the product being purified by column chromatography. Various n-doxyl phosphocholines have been recently used as biophysical tools to elucidate membrane trafficking with phosphatidylinositol transfer proteins and as fluorescent quenchers in lipid bilayer structural studies.
Phosphatidylcholine (PC), a strong bilayer-forming lipid is the most common phospholipid in mammalian membranes. The excretory and secretory products of helminths has a small hapten-like portion called phosphorylcholine (PC). The 5th carbon of the sn-2 stearic acid chain of 1-palmitoyl-2-stearoyl-(5-doxyl)-sn-glycero-3-phosphocholine analog has a Doxyl PC, a spin probe attached to it covalently.
Application
16:0-5 Doxyl PC may be used:
- as a component in virus-like large unilamellar vesicles (VL LUVs) to quench 4-chloro-7-nitrobenz-2-oxa-1,3-diazole (NBD) fluorescence emission
- in the preparation of multi-lamellar vesicles (MLVs) as a site-specific quencher to perform fluorescence quenching studies
- in the preparation of spin-labelled multi-lamellar vesicles (MLVs)
Actions biochimiques/physiologiques
Phosphatidylcholine (PC) lowers the levels of cholesterol and triglycerides.
Conditionnement
5 mL Clear Glass Sealed Ampule (810601P-1mg)
Notes préparatoires
Product use: To prevent aggregation, prepare water-based solutions of 2 mM stock solutions of n-DOXYL PCs and store in plastic. Dilute stock solutions to 0.03- 0.1 mM solutions for EPR studies. For liposome preparations in fluorescent quenching measurements, dissolve the doxyl lipid in 150 μl absolute ethanol for a concentration of 40.3 mM , Additional supplemental information.
Informations légales
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Souvent commandé avec ce produit
Code de la classe de stockage
11 - Combustible Solids
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
The Membranes of Cells (2016)
Host defenses to helminths
Clinical Immunology, 367-377 (2013)
Diego E Sastre et al.
The Journal of biological chemistry, 295(7), 2136-2147 (2019-12-05)
PlsX plays a central role in the coordination of fatty acid and phospholipid biosynthesis in Gram-positive bacteria. PlsX is a peripheral membrane acyltransferase that catalyzes the conversion of acyl-ACP to acyl-phosphate, which is in turn utilized by the polytopic membrane
H C Chan et al.
Magnetic resonance in medicine, 8(2), 160-170 (1988-10-01)
The positively charged nitroxide spin label, 2,2,6,6-tetramethyl-piperidine-N-oxyl-4-trimethylammonium (Cat1), was encapsulated in two types of liposomes, phosphatidylserine/phosphatidylcholine (PS/PC) and phosphatidylserine/distearoylphosphatidylcholine/dipalmitoylphosphatidyl choline (PS/DSPC/DPPC). The liposomes were incubated with mouse thymus-bone marrow (TB) cells to study the uptake and metabolism of nitroxides entrapped
Fayi Wu et al.
Biochemistry, 45(41), 12510-12518 (2006-10-13)
The putative substrate-binding site in lipoxygenases is long and internal. There is little direct evidence about how the unsaturated fatty acid substrates enter and move within the cavity to position themselves correctly for electron transfer reactions with the catalytic non-heme
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique