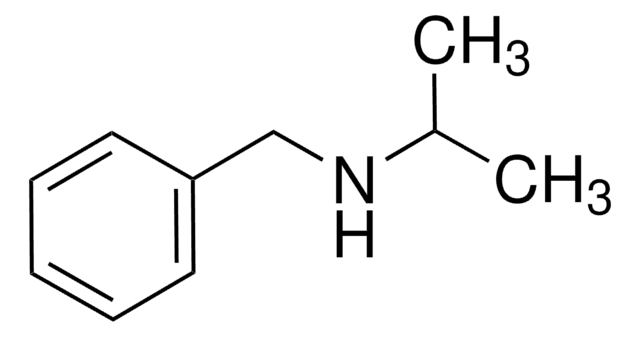
D34108
Dibenzylamine
97%
Synonyme(s) :
(N-Benzylaminomethyl)benzene, Bibenzylamine, DBA, Dibenzylamine (8CI), N,N-Dibenzylamine, N-(Phenylmethyl)benzenemethanamine, N-Benzyl-1-phenylmethanamine, N-Benzylbenzylamine
About This Item
Produits recommandés
Pureté
97%
Forme
liquid
Indice de réfraction
n20/D 1.574 (lit.)
Point d'ébullition
300 °C (lit.)
Pf
−26 °C (lit.)
Densité
1.026 g/mL at 25 °C (lit.)
Chaîne SMILES
C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H15N/c1-3-7-13(8-4-1)11-15-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2
Clé InChI
BWLUMTFWVZZZND-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Environmental monitoring in shale gas wastewater: Dibenzylamine was identified among the hazardous substances in shale gas wastewater, with research characterizing its concentration and distribution in the Upper Yangtze River, contributing to improved environmental management practices (Tang et al., 2024).
- Advancements in organic synthesis: Dibenzylamine was used in a novel synthetic strategy for (L)-Monomethyl Tyrosine via bulky ′forced-traceless′ regioselective Pd-catalyzed C(sp(2))-H activation, showcasing its utility in pharmaceutical compound development (Illuminati et al., 2023).
- Application in crystallography: The crystal structure of di-benzyl-ammonium was elucidated, providing insights into molecular interactions and potential applications in material science and drug design (Traoré et al., 2023).
- Utilization in green chemistry: Dibenzylamine facilitated a green approach towards Triazole forming reactions, aiming to develop anticancer drugs by minimizing environmental impact and enhancing reaction efficiency (Rastogi et al., 2023).
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
289.4 °F - closed cup
Point d'éclair (°C)
143 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique