656631
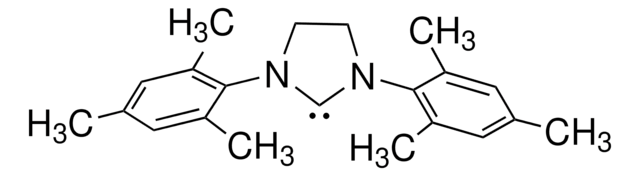
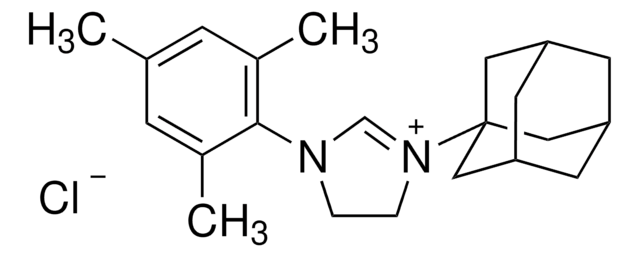
1,3-Bis(2,4,6-trimethylphenyl)imidazolinium chloride
95%
Synonyme(s) :
1,3-Dimesitylimidazolidinium chloride, 4,5-Dihydro-1,3-bis(2,4,6-trimethylphenyl)-1H-imidazolium chloride, 4,5-Dihydro-1,3-dimesityl-1H-imidazolium chloride, N,N′-(2,4,6-Trimethylphenyl)dihydroimidazolium chloride
About This Item
Produits recommandés
Pureté
95%
Pertinence de la réaction
reagent type: ligand
Pf
280-286 °C
Chaîne SMILES
[Cl-].Cc1cc(C)c(N2CC[N+](=C2)c3c(C)cc(C)cc3C)c(C)c1
InChI
1S/C21H27N2.ClH/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;/h9-13H,7-8H2,1-6H3;1H/q+1;/p-1
Clé InChI
COGMCBFILULEOS-UHFFFAOYSA-M
Catégories apparentées
Description générale
Application
Precursor to an N-heterocyclic carbene catalysts used for:
- A regioselective cycloadditon of terminal acetylenes with azides leading to 1,4-disubstitutedtriazoles. Internal acetylenes can also be used with this catalyst.
- Markovnikov-type hydration of terminal alkynes
- Hydrosilylation of ketones and cycloaddition of azides and alkynes
- Suzuki-Miyaura reactions
- Luminescence experiments
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Metal complex-catalyzed cross-coupling reactions of unactivated substrates introduce diverse phosphine ligands in chemical marketplace.
Emerging class of privileged ligands
A wide range of NHC ligands are commonly available which exhibit high activities.
The Hazari group has developed an improved palladium precatalyst scaffold for a wide range of cross-coupling reactions
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![Chloro[1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]copper(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/160/888/97509eeb-0719-4853-aaae-8a9d02f4f7ad/640/97509eeb-0719-4853-aaae-8a9d02f4f7ad.png)
![[1,3-Bis(2,6-diisopropylphenyl)-imidazol-2-ylidene]copper(I) chloride](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)