P5511
Protein Kinase A from bovine heart
≥0.8 units/μg protein, lyophilized powder
Synonym(s):
Protein Kinase, 3′,5′-cyclic-AMP-dependent from bovine heart
Sign Into View Organizational & Contract Pricing
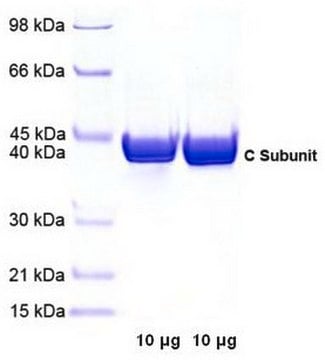
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
eCl@ss:
32160410
NACRES:
NA.77
Recommended Products
form
lyophilized powder
specific activity
≥0.8 units/μg protein
composition
Protein, ≥70%
storage temp.
−20°C
Gene Information
cow ... PRKACA(282322) , PRKACB(282323)
Looking for similar products? Visit Product Comparison Guide
General description
Protein Kinase A (PKA) is a serine/threonine kinase, which exists as a tetrameric holoenzyme.
Application
Protein Kinase A from bovine heart has been used to determine its activity using a molecular beacon probe. It has also been used to study the interaction of staurosporine with the ATP-binding site of kinases.
Protein Kinase A from bovine heart has been used to inhibit the phosphorylation of myosin.
Biochem/physiol Actions
Protein Kinase A (PKA) inhibits hormone-sensitive lipase translocation from cytosol to storage droplets and blocks lipolysis. It regulates apoptosis, mitochondrial respiration and ATP synthesis. PKA is modulated by protease calpain.
Many 3′,5′-cyclic AMP dependent protein kinases have been reported. Structural studies (Traugh, J.A., et al., Meth. Enzymol., 38, 290 [1974]) show the presence of at least two subunits, the regulatory subunit and the catalytic subunit. When both units are linked together, the catalytic activity is inhibited. However, when the cyclic-AMP binds to the regulatory subunit, the catalytic subunit is released and can then catalyze the transfer of phosphate from ATP to various proteins.
Packaging
Package size based on protein content
Unit Definition
One unit will transfer 1.0 picomole phosphate from ATP to hydrolyzed and partially dephosphorylated casein per minute at pH 6.5 at 30°C in the presence of cyclic AMP, determined by measuring the production of ADP.
Physical form
Crude lyophilized powder containing EDTA and potassium phosphate, pH 7.5.
Preparation Note
Fractionated essentially by procedure of Gilman, A., Proc. Natl. Acad. Sci. USA, 67, 305 (1970).
Analysis Note
Protein determined by biuret.
inhibitor
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alkylation of Staurosporine to Derive a Kinase Probe for Fluorescence Applications
Dr. Alexandar
Chemistry of Materials (2016)
Simple fluorescence-based detection of protein kinase A activity using a molecular beacon probe
Journal of Bioenergetics and Biomembranes (2017)
Cardiomyocyte Hypocontractility and Reduced Myofibril Density in End-Stage Pediatric Cardiomyopathy.
Ilse A E Bollen et al.
Frontiers in physiology, 8, 1103-1103 (2018-01-10)
Dilated cardiomyopathy amongst children (pediatric cardiomyopathy, pediatric CM) is associated with a high morbidity and mortality. Because little is known about the pathophysiology of pediatric CM, treatment is largely based on adult heart failure therapy. The reason for high morbidity
Signaling the signal: Protein Kinase a (PKA) inhibition by insulin-formed H2O2 and reactivation by thioredoxin
de Pina MZ, et al.
The Journal of Biological Chemistry (2008)
Probes of the mitochondrial cAMP-dependent protein kinase
Shell JR and Lawrence DS
Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1834(7), 1359-1363 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service