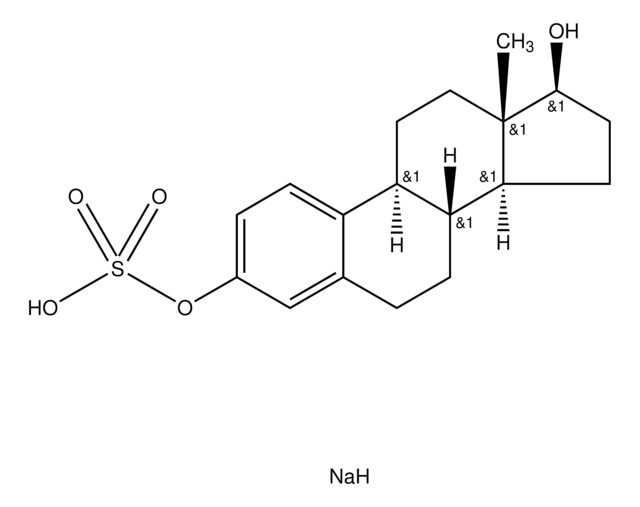
E9645
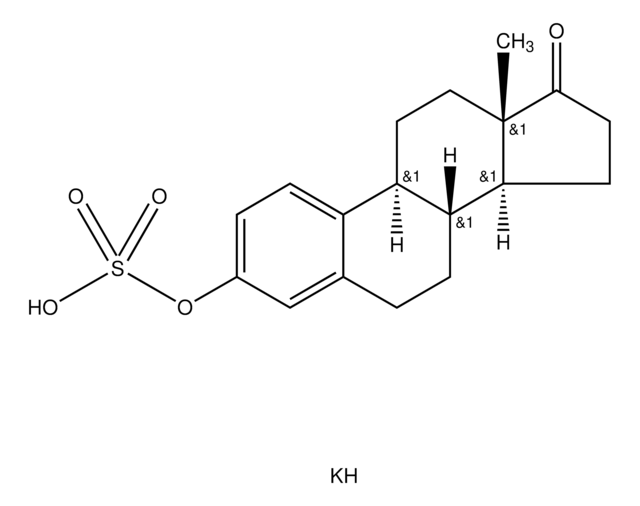
Estrone sulfamate
≥98%
Synonym(s):
1,3,5(10)-Estratrien-17-one 3-sulfamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H23NO4S
CAS Number:
Molecular Weight:
349.44
MDL number:
UNSPSC Code:
12352202
NACRES:
NA.77
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98%
form
powder
storage temp.
−20°C
SMILES string
[S](=O)(=O)(N)Oc1cc2c(cc1)[C@@H]3[C@H]([C@H]4[C@](CC3)(C(=O)CC4)C)CC2
InChI
1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1
InChI key
RVKFQAJIXCZXQY-CBZIJGRNSA-N
Application
Lead compound for design of steroid sulfatase inhibitors.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Purohit et al.
Biochemistry, 34(36), 11508-11514 (1995-09-12)
Steroid sulfatases are responsible for the hydrolysis of 3beta-hydroxy steroid sulfates, such as cholesterol and pregnenolone sulfate, and have an important role in regulating the synthesis of estrogenic steroids, from estrone sulfate and dehydroepiandrosterone sulfate, in endocrine-dependent tumors. Although little
Active site directed inhibition of estrone sulfatase by nonsteroidal coumarin sulfamates.
L W Woo et al.
Journal of medicinal chemistry, 39(7), 1349-1351 (1996-03-29)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service