32091
Sulfamonomethoxine
VETRANAL®, analytical standard
Synonym(s):
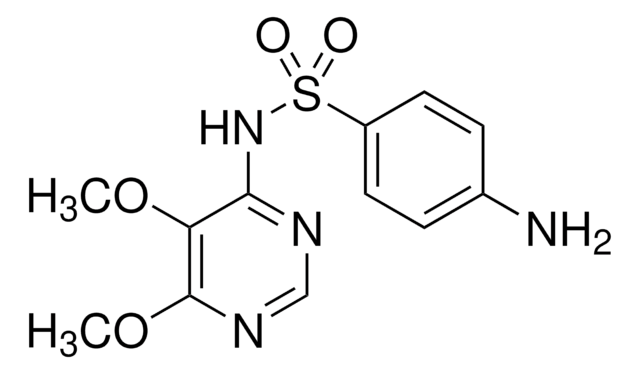
4-Amino-N-(6-methoxy-4-pyrimidinyl)benzenesulfonamide, 4-Methoxy-6-sulfanilamidopyrimidine, N1-(6-Methoxy-4-pyrimidinyl)sulfanilamide, SMM
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
VETRANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
impurities
≤3.0% water
application(s)
clinical testing
format
neat
SMILES string
COc1cc(NS(=O)(=O)c2ccc(N)cc2)ncn1
InChI
1S/C11H12N4O3S/c1-18-11-6-10(13-7-14-11)15-19(16,17)9-4-2-8(12)3-5-9/h2-7H,12H2,1H3,(H,13,14,15)
InChI key
WMPXPUYPYQKQCX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Tissues, milk and eggs of food-producing animals by ultrasound-assisted extraction (UAE) and liquid chromatography combined with tandem mass spectrometry (LC-MS/MS).
- Food samples of animal origin by QuEChERS (quick, easy, cheap, effective, rugged and safe) extraction procedure, SOSLE (salting-out supported liquid extraction) method and nanoflow liquid chromatography high resolution mass spectrometry (LC-HRMS).
- Bovine muscles by hydrophilic interaction liquid chromatography (HILIC) combined with LC-MS/MS.
- Commercial eggs by liquid-liquid extraction (LLE), hybrid solid-phase extraction (SPE) and LC-MS/MS.
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service