742945
TurboBeads™ TEMPO
≥99%
Synonym(s):
Nano particles, magnetic, TEMPO functionalized
About This Item
Recommended Products
product line
TurboBeads™
Assay
≥99%
form
powder
composition
carbon content, ≤14 wt. %
reaction suitability
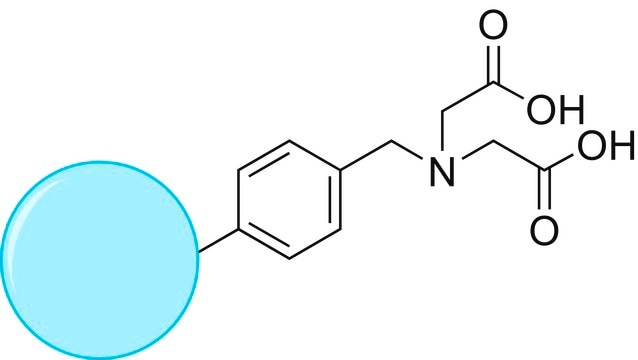
reaction type: solution phase peptide synthesis
reactivity: alcohol reactive
extent of labeling
≥0.1 mmol/g loading (TEMPO)
magnetization
≥120 emu/g, mass saturation
surface area
≥15 m2/g
average diameter
≤50 nm
suitability
conforms to structure for Infrared spectrum
Application
Packaging
Analysis Note
air-stability:
weight gain in air at 400°C >20 wt.%
weight gain in air at 100°C <3 wt.%
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service