All Photos(1)
About This Item
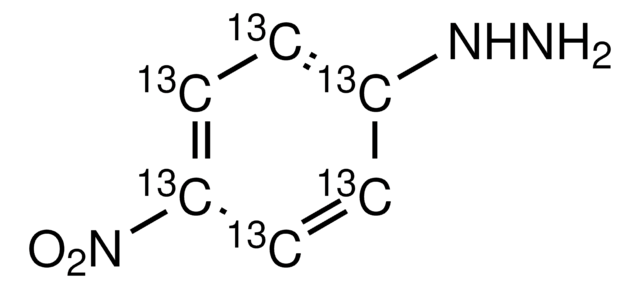
Linear Formula:
O2NC6H4NHNH2
CAS Number:
Molecular Weight:
153.14
Beilstein:
608107
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
96%
form
solid
contains
≥30% water as stabilizer
mp
156 °C (dec.) (lit.)
SMILES string
NNc1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H7N3O2/c7-8-5-1-3-6(4-2-5)9(10)11/h1-4,8H,7H2
InChI key
KMVPXBDOWDXXEN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Expl. 1.1 - Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
1 - Explosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Steinebach et al.
Analytical biochemistry, 230(1), 159-166 (1995-09-01)
Pig kidney diamine oxidase was purified to homogeneity. The reaction product of the cofactor with p-nitrophenylhydrazine (pNPH) was liberated with pronase treatment and purified. 1H NMR, uv/vis, and electrospray tandem mass spectroscopy revealed it to be a dipeptide with the
I Frébort et al.
European journal of biochemistry, 225(3), 959-965 (1994-11-01)
Interactions of two distinct quinoprotein amine oxidases from Aspergillus niger, AO-I and AO-II, with active-site covalent modifiers have been investigated. Both enzymes are inhibited similarly by phenylhydrazine or p-nitrophenylhydrazine, forming an orange Schiff base with a carbonyl group of topaquinone
Minae Mure et al.
Journal of the American Chemical Society, 125(20), 6113-6125 (2003-06-06)
4-n-Butylamino-5-ethyl-1,2-benzoquinone (1(ox)) has been synthesized as a model compound for the LTQ (lysine tyrosyl quinone) cofactor of lysyl oxidase (LOX). At pH 7, 1(ox) has a lambda(max) at 504 nm and exists as a neutral o-quinone in contrast to a
N Nakamura et al.
The Journal of biological chemistry, 271(9), 4718-4724 (1996-03-01)
Resonance Raman spectroscopy is an excellent technique for providing structural information on the 2,4, 5-trihydroxyphenylalanine quinone (TPQ) cofactor in copper-containing amine oxidases. This technique has been used to investigate the copper- and O2-dependent biosynthesis of the TPQ cofactor in phenylethylamine
Y Kariya et al.
Journal of biochemistry, 123(2), 240-246 (1998-04-16)
Compositional analyses of heparin (Hep) and heparan sulfate (HS) have been undertaken with disaccharide units obtained by either enzymatic digestion with heparitinases or hydrazinolysis/deamination reaction of polysaccharides. Unsaturated disaccharide units generated by the enzymatic method are detectable on HPLC with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service