524700
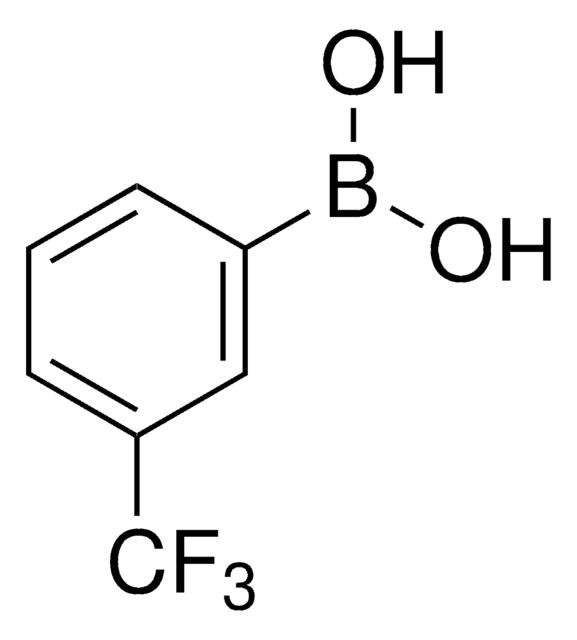
3,4,5-Trifluorophenylboronic acid
≥95%
Synonym(s):
3,4,5-Trifluorobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F3C6H2B(OH)2
CAS Number:
Molecular Weight:
175.90
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder
mp
290-295 °C (lit.)
SMILES string
OB(O)c1cc(F)c(F)c(F)c1
InChI
1S/C6H4BF3O2/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,11-12H
InChI key
UHDDEIOYXFXNNJ-UHFFFAOYSA-N
Application
Reactant involved in:
- Preparation of phenylboronic catechol esters and determination of Lewis acidity
- Synthesis of benzopyranone derivatives as GABAA receptor modulators
- Synthesis of multisubstituted olefins and conjugate dienes
- Suzuki cross-coupling reactions
- Preparation of fluorinated aromatic poly(ether-amide)s
Other Notes
Contains varying amounts of anhydride.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mikhail Y Vorona et al.
Materials (Basel, Switzerland), 13(8) (2020-04-26)
Anthracene-based semiconductors have attracted great interest due to their molecular planarity, ambient and thermal stability, tunable frontier molecular orbitals and strong intermolecular interactions that can lead to good device field-effect transistor performance. In this study, we report the synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service