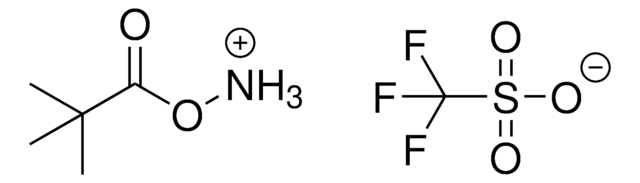All Photos(1)
About This Item
Linear Formula:
C6H5CH2ONHCO2C(CH3)3
CAS Number:
Molecular Weight:
223.27
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
99%
mp
45-47 °C (lit.)
SMILES string
CC(C)(C)OC(=O)NOCc1ccccc1
InChI
1S/C12H17NO3/c1-12(2,3)16-11(14)13-15-9-10-7-5-4-6-8-10/h4-8H,9H2,1-3H3,(H,13,14)
InChI key
MZNBNPWFHGWAGH-UHFFFAOYSA-N
General description
tert-Butyl N-(benzyloxy)carbamate (tert-butyl benzyloxycarbamate), a protected hydroxylamine, is an N-alkyl-N-benzyloxy carbamate. Its C-N cross coupling reaction with fluorescein ditriflate has been reported. It participates in facile intramolecular cyclization with various carbon nucleophiles to afford functionalized 5- and 6-membered protected cyclic hydroxamic acids.
Application
tert-Butyl N-(benzyloxy)carbamate was used in the preparation of seven-membered cyclic hydroxamic acids. It may be used in the synthesis of 2-(N-formyl-N-hydroxyamino) ethylphosphonate (IPP).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of rhodamines from fluoresceins using Pd-catalyzed C-N cross-coupling.
Grimm JB and Lavis LD.
Organic Letters, 13(24), 6354-6357 (2011)
Yuan Liu et al.
Tetrahedron, 67(12), 2206-2214 (2011-04-19)
N-Alkyl-N-benzyloxy carbamates, 2, undergo facile intramolecular cyclization with a variety of carbon nucleophiles to give functionalized 5- and 6-membered protected cyclic hydroxamic acids, 3, in good to excellent yields. This method can be extended to prepare seven-membered cyclic hydroxamic acids
I Kursula et al.
European journal of biochemistry, 268(19), 5189-5196 (2001-10-09)
The crystal structure of leishmania triosephosphate isomerase (TIM) complexed with 2-(N-formyl-N-hydroxy)-aminoethyl phosphonate (IPP) highlights the importance of Asn11 for binding and catalysis. IPP is an analogue of the substrate D-glyceraldehyde-3-phosphate, and it is observed to bind with its aldehyde oxygen
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service