291919
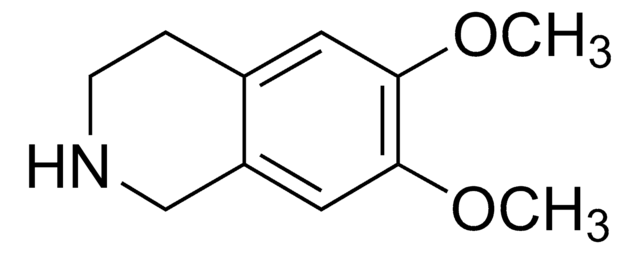
6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H15NO2 · HCl
CAS Number:
Molecular Weight:
229.70
Beilstein:
3634126
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
260-265 °C (lit.)
solubility
soluble 25 mg/mL, clear, colorless (1N NaOH in methanol)
SMILES string
Cl.COc1cc2CCNCc2cc1OC
InChI
1S/C11H15NO2.ClH/c1-13-10-5-8-3-4-12-7-9(8)6-11(10)14-2;/h5-6,12H,3-4,7H2,1-2H3;1H
InChI key
SHOWAGCIRTUYNA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride is also referred as heliamine. It is an alkaloid isolated from mexican cereoid, Backebergia militaris.
Application
6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride has been used as starting material for the synthesis of more complex isoquinolines and quinolizidines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Glusa et al.
Die Pharmazie, 45(6), 408-410 (1990-06-01)
The synthesis of the title compounds starting from 2-Chlormethylbenzdioxan and Tetrahydroisochinolines is presented. Their actions on the platelet aggregation and the inhibition of alpha-adrenoceptors at the isolated rabbit aorta and the vas deferens of the guinea pig were investigated.
R Mata et al.
Journal of pharmaceutical sciences, 69(1), 94-95 (1980-01-01)
Backebergia militaris (Andot) Bravo ex Sánchez Mejorada yielded alkaloid crystals from a fractionated ethanol extract of only 20 g of plant material. The alkaloid was identified as heliamine (6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline) hydrochloride. A second alkaloid, 3,4-dimethoxy-beta-phenethylamine hydrochloride, was crystallized after preparative TLC
Heterocycles, 34, 1857-1857 (1992)
S A Barker et al.
Biochemical pharmacology, 30(17), 2461-2468 (1981-09-01)
Gas chromatographic/mass spectrometric data are presented which demonstrate the presence of 6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (DHTIQ) as a normal constituent of rat brain. The level of DHTIQ was calculated to be 10.0 +/- 3.0 ng/g wet weight (+/- S.D., N = 9) of
Ken-ichi Umehara et al.
Drug metabolism and disposition: the biological fate of chemicals, 37(7), 1427-1433 (2009-04-11)
(-)-N-{2-[(R)-3-(6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)piperidino]ethyl}-4-fluorobenzamide (YM758), a novel "funny" If current channel (If channel) inhibitor, is developed as a treatment for stable angina and atrial fibrillation. In this study, the pharmacokinetic/pharmacodynamic (PK/PD) relationship after intravenous administration of YM758 to tachycardia-induced dogs was investigated and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service