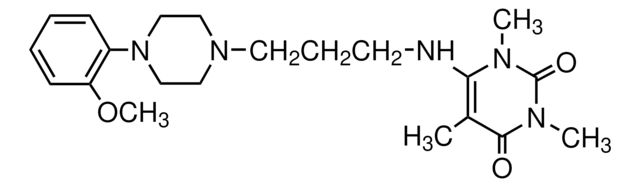B134
BMY 7378 dihydrochloride
≥98% (HPLC), solid
Synonym(s):
8-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride
About This Item
Recommended Products
Assay
≥98% (HPLC)
form
solid
color
white
solubility
H2O: >5 mg/mL
SMILES string
Cl.Cl.COc1ccccc1N2CCN(CC2)CCN3C(=O)CC4(CCCC4)CC3=O
InChI
1S/C22H31N3O3.2ClH/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22;;/h2-3,6-7H,4-5,8-17H2,1H3;2*1H
InChI key
NIBOMXUDFLRHRV-UHFFFAOYSA-N
Gene Information
human ... ADRA1D(146) , HTR1A(3350)
Biochem/physiol Actions
Features and Benefits
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Review alpha 1 adrenoceptors as well as their agonists, antagonists, and tissue expression patterns. We suggest several modulators and alternatives for working with a-1 adrenoreceptors.
Review alpha 1 adrenoceptors as well as their agonists, antagonists, and tissue expression patterns. We suggest several modulators and alternatives for working with a-1 adrenoreceptors.
Review alpha 1 adrenoceptors as well as their agonists, antagonists, and tissue expression patterns. We suggest several modulators and alternatives for working with a-1 adrenoreceptors.
Review alpha 1 adrenoceptors as well as their agonists, antagonists, and tissue expression patterns. We suggest several modulators and alternatives for working with a-1 adrenoreceptors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service