D2757
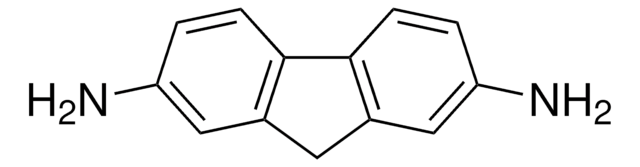
2,3-Diaminonaphthalene
≥95% purity (HPLC), powder
Synonym(s):
2,3-Naphthalenediamine, DAN
About This Item
Recommended Products
product name
2,3-Diaminonaphthalene, ≥95% (HPLC), powder
Quality Level
Assay
≥95% (HPLC)
form
powder
color
off-white to dark beige, to Dark Brown
mp
198-200 °C
solubility
pyridine: 50 mg/mL
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Nc1cc2ccccc2cc1N
InChI
1S/C10H10N2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6H,11-12H2
InChI key
XTBLDMQMUSHDEN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- 2,3-Diaminonaphthalene (DAN) has been used for the fluorometric measurement of nitrite/nitrate.
- DAN has been used to develop a solid phase extraction-multisyringe flow injection system to spectrophotometrically detect the presence of selenium.
- It has been employed in a study to develop a colorimetric assay for the detection of methylglyoxal.
- It has also been used in a study to develop a novel ratiometric fluorescent probe for the in-situ detection of alkaline phosphatase activity.
Biochem/physiol Actions
DAN also reacts with selenite to form 4,5-benzopiazselenol which is detectable via absorptiometry or fluorometry.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1A - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service