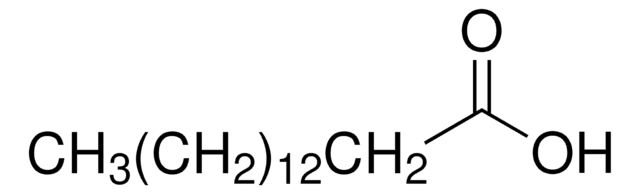70082
Myristic acid
≥98.0% (GC)
Synonym(s):
1-Tridecanecarboxylic acid, C14:0, NSC 5028, Tetradecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)12COOH
CAS Number:
Molecular Weight:
228.37
Beilstein:
508624
EC Number:
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (GC)
form
powder
bp
250 °C/100 mmHg (lit.)
mp
52-54 °C (lit.)
53-56 °C
functional group
carboxylic acid
lipid type
saturated FAs
shipped in
ambient
storage temp.
room temp
SMILES string
CCCCCCCCCCCCCC(O)=O
InChI
1S/C14H28O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14(15)16/h2-13H2,1H3,(H,15,16)
InChI key
TUNFSRHWOTWDNC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
<ul>
<li><strong>Safety assessment of myristic acid as a food ingredient.</strong> Myristic acid was evaluated for its safety as a food ingredient, demonstrating its compliance with health and safety standards and supporting its use in food manufacturing (Burdock and Carabin, 2007).</li>
<li><strong>Example of fatty acid-loaded lipoplex in enhancing in vitro gene transfer efficacies of cationic amphiphile.</strong> This research outlines how myristic acid can be used to improve the efficiency of gene delivery systems in medical research, suggesting a pivotal role in enhancing transfection efficiencies (Majeti et al., 2005).</li>
</ul>
<li><strong>Safety assessment of myristic acid as a food ingredient.</strong> Myristic acid was evaluated for its safety as a food ingredient, demonstrating its compliance with health and safety standards and supporting its use in food manufacturing (Burdock and Carabin, 2007).</li>
<li><strong>Example of fatty acid-loaded lipoplex in enhancing in vitro gene transfer efficacies of cationic amphiphile.</strong> This research outlines how myristic acid can be used to improve the efficiency of gene delivery systems in medical research, suggesting a pivotal role in enhancing transfection efficiencies (Majeti et al., 2005).</li>
</ul>
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dale D O Martin et al.
Biochimie, 93(1), 18-31 (2010-11-09)
Myristoylation corresponds to the irreversible covalent linkage of the 14-carbon saturated fatty acid, myristic acid, to the N-terminal glycine of many eukaryotic and viral proteins. It is catalyzed by N-myristoyltransferase. Typically, the myristate moiety participates in protein subcellular localization by
Yanhong Hao et al.
iScience, 24(9), 102974-102974 (2021-08-17)
Asymptomatic infection is a big challenge in curbing the spread of COVID-19. However, its identification and pathogenesis elucidation remain issues. Here, by performing comprehensive lipidomic characterization of serum samples from 89 asymptomatic COVID-19 patients and 178 healthy controls, we screened
Katarzyna Reczyńska et al.
Lipids, 55(2), 117-126 (2020-01-24)
The impact of saturated fatty acids (FA) on viability and properties of malignant and nonmalignant cells has not been studied in detail so far. The present study was aimed at evaluation of the influence of saturated FA (10:0-18:0) on malignant
Dalit Shental-Bechor et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(44), 17839-17844 (2012-08-01)
We present an integrated experimental and computational study of the molecular mechanisms by which myristoylation affects protein folding and function, which has been little characterized to date. Myristoylation, the covalent linkage of a hydrophobic C14 fatty acyl chain to the
Anja Meier et al.
Hepatology (Baltimore, Md.), 58(1), 31-42 (2012-12-06)
Chronic infection with the human hepatitis B virus (HBV) is a global health problem and a main cause of progressive liver diseases. HBV exhibits a narrow host range, replicating primarily in hepatocytes. Both host and hepatocyte specificity presumably involve specific
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service