AG938
Synuclein, α, recombinant human
Synonym(s):
α-Synuclein protein, Alpha Synuclein
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
eCl@ss:
32160405
NACRES:
NA.41
Recommended Products
biological source
human
Quality Level
Assay
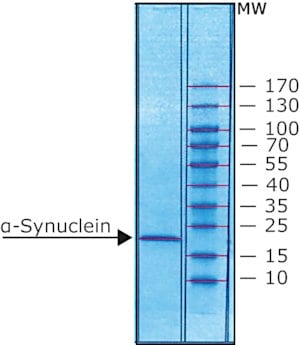
>95% (gel scan and mass spectrometry)
form
solid
shelf life
12 mo.
manufacturer/tradename
Chemicon®
solubility
water: slightly soluble
suitability
suitable for molecular biology
NCBI accession no.
UniProt accession no.
Gene Information
human ... SNCA(6622)
General description
Research area: Neuroscience
α-Synuclein, a member of the synuclein family of proteins that localize to presynaptic terminals under physiological conditions. It is comprised of three primary regions: an amino terminus containing apolipoprotein lipid-binding motifs, which are anticipated to form amphiphilic helices. The central region, known as non-Aβ component (NAC) containing a hydrophobic domain that gives β-sheet potential, and the carboxyl terminus, characterized by a high negative charge and remains unstructured. Under physiological conditions, members of the synuclein family, including α-synuclein, are predominantly neuronal proteins.
α-Synuclein, a member of the synuclein family of proteins that localize to presynaptic terminals under physiological conditions. It is comprised of three primary regions: an amino terminus containing apolipoprotein lipid-binding motifs, which are anticipated to form amphiphilic helices. The central region, known as non-Aβ component (NAC) containing a hydrophobic domain that gives β-sheet potential, and the carboxyl terminus, characterized by a high negative charge and remains unstructured. Under physiological conditions, members of the synuclein family, including α-synuclein, are predominantly neuronal proteins.
Application
Synuclein, α, recombinant human has been used:
- for preparation of α-synuclein fibrils to access the SAR228810 antibody against the risk of amyloid-related imaging abnormalities (ARIA).
- to study the formation of isoasparate protein damage responsible to trigger familial Parkionsonian phenotypes.
- to study its interaction with iron and copper, in order to understand their role in proteinopathies.
Biochem/physiol Actions
Synuclein, α is the principal component of Lewy bodies and a pathological hallmark of synucleinopathies, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). The deposition of αS aggregates is a defining characteristic of all these diseases.
Physical form
Lyophilized from TBS. Resuspend in water at a concentration of 1 mg/mL.
Storage and Stability
Maintain lyophilized material at -20ºC for up to 12 months after date of receipt. After reconstitution maintain at -20ºC to -70ºC for up to 2 weeks in undiluted aliquots. Avoid freeze/thaw cycles to avoid aggregation.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The many faces of ?-synuclein: from structure and toxicity to therapeutic target
Lashuel HA, et al.
Nature Reviews. Neuroscience, 14, 38-48 (2013)
Emerging Approaches to Investigate the Influence of Transition Metals in the Proteinopathies
Lermyte F, et al.
Cells, 8(10), 1231-1231 (2019)
Alpha-synuclein structure and Parkinson?s disease ? lessons and emerging principles
Meade RM, et al.
Mol. Neurodegener., 14 (2019)
?-Synuclein in Parkinson's Disease
Stefanis L
Cold Spring Harbor Perspectives in Medicine, 2(2), a009399-a009399 (2012)
Molecular ageing of alpha- and Beta-synucleins: protein damage and repair mechanisms
Vigneswara V, et al.
PLoS ONE, 8(4), e61442-e61442 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service