All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O
CAS Number:
Molecular Weight:
110.11
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
70-74 °C/1 mmHg (lit.)
mp
36-39 °C (lit.)
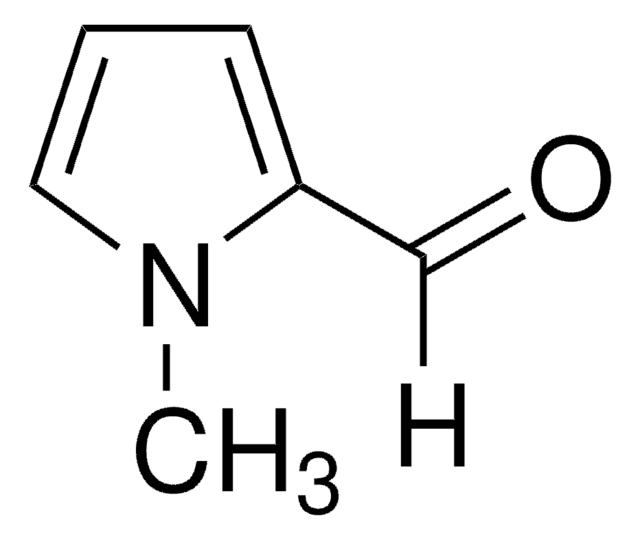
SMILES string
[H]C(=O)c1nccn1C
InChI
1S/C5H6N2O/c1-7-3-2-6-5(7)4-8/h2-4H,1H3
InChI key
UEBFLTZXUXZPJO-UHFFFAOYSA-N
General description
1-Methyl-2-imidazolecarboxaldehyde is a heterocyclic building block. It affords tripodal ligands on condensation reaction with tris-(2-aminoethyl)amine (tren). These tripodal ligands react with iron(III) salts in the presence of air to afford iron(II) complexes. Its vibrational spectral studies have been reported.
Application
1-Methyl-2-imidazolecarboxaldehyde may be used in the synthesis of:
- Schiff bases
- ligand N,N-dimethyl-N′-(1-methylimidazole-2-ylmethyl)-ethylenediamine
- [(bis(1-methylimidazol-2-yl)methyl)(2-(pyridyl-2-yl)ethyl)amine]
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
DFT, FT-IR and FT-Raman investigations of 1-methyl-2-imidazolecarboxaldehyde.
Polat T and Yurdakul S.
Journal of Molecular Structure, 1053, 27-37 (2013)
Structural, spectroscopic and redox studies of a new ruthenium (III) complex with an imidazole-rich tripodal ligand.
Scarpellini M, et al.
Inorgorganica Chimica Acta, 357(3), 707-715 (2004)
Synthesis, crystal structure and redox properties of bis-imidazolyl-containing copper (II) complexes.
Musie GT, et al.
Inorgorganica Chimica Acta, 348, 69-74 (2003)
Synthesis and characterization of tripodal iron (II) complexes prepared from 2-pyridinecarboxaldehyde and 1-methyl-2-imidazolecarboxaldehyde: stabilization of iron (II) cations with N 6 donor sets.
Brewer G, et al.
Inorgorganica Chimica Acta, 357(8), 2390-2396 (2004)
Iron (III) complexes of sterically hindered tetradentate monophenolate ligands as functional models for catechol 1, 2-dioxygenases: The role of ligand stereoelectronic properties.
Velusamy M, et al.
Inorganic Chemistry, 43(20), 6284-6293 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service