All Photos(1)
About This Item
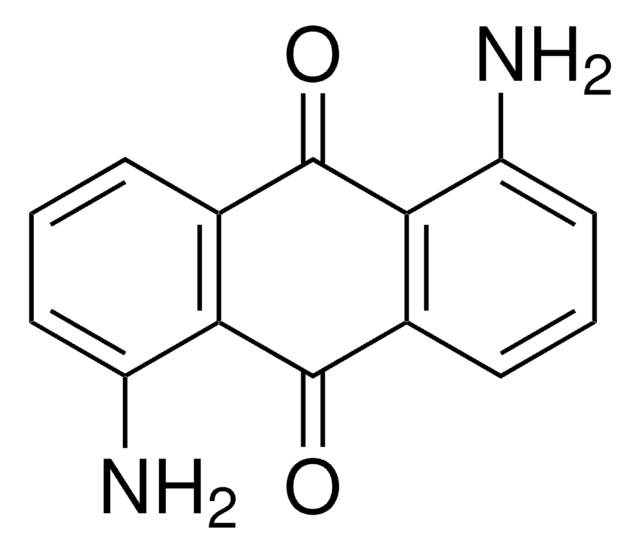
Empirical Formula (Hill Notation):
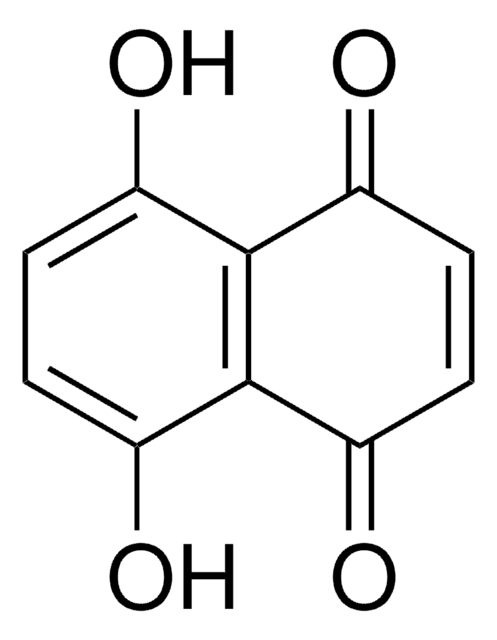
C18H10O4
CAS Number:
Molecular Weight:
290.27
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
350-351 °C (lit.)
SMILES string
Oc1c2C(=O)c3ccccc3C(=O)c2c(O)c4ccccc14
InChI
1S/C18H10O4/c19-15-9-5-1-2-6-10(9)16(20)14-13(15)17(21)11-7-3-4-8-12(11)18(14)22/h1-8,19-20H
InChI key
QECAURYYBPUIFF-UHFFFAOYSA-N
Related Categories
General description
Crystallographic analysis of 6,11-dihydroxy-5,12-naphthacenedione has been reported. Study indicates the presence of (3Z,3′Z)-3,3′-(ethane-1,2-diylidene)bis[isobenzofuran-1(3H)-one] as impurity.
Application
6,11-Dihydroxy-5,12-naphthacenedione was used in the synthesis of a tetracene derivative, 5,6,11,12-tetrachlorotetracene. It was also used for the synthesis of (3Z,3′Z)-3,3′-(ethane-1,2-diylidene)bis[isobenzofuran-1(3H)-one]. It may be used for the following studies:
- Synthesis of self-assembled, chair-shaped dirhenium(I) macrocyclic compounds.
- Synthesis of half-sandwich Ir, Rh-based organometallic molecular boxes.
- As ligand for the synthesis of supramolecular coordination complexes (SCCs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
5, 6, 11, 12-Tetrachlorotetracene, a tetracene derivative with p-stacking structure: The synthesis, crystal structure and transistor properties.
Chi X, et al.
Organic Electronics, 9(2), 234-240 (2008)
Ying-Feng Han et al.
Dalton transactions (Cambridge, England : 2003), 39(16), 3976-3984 (2010-04-08)
Reactions of [Cp*MCl(mu-Cl)](2) (M = Ir or Rh) with 6,11-dihydroxy-5,12-naphthacenedione (H(2)DHNA) in the presence of base, gave the corresponding binuclear complexes [Cp*(2)M(2)(mu-DHNA)Cl(2)] (M = Ir (1a); M = Rh (1b)), respectively. Treatment of 1a or 1b with bidentate ligands (L)
Synthesis of rhenium-based M 2 LL'-type supramolecular coordination complexes from flexible ligands.
Shankar B, et al.
Journal of Organometallic Chemistry, 743, 109-113 (2013)
(3Z, 3'Z)-3, 3'-(Ethane-1, 2-diylidene) bis [isobenzofuran-1 (3H)-one].
Ono K, et al.
Acta Crystallographica Section E, Structure Reports Online, 65(9), 2118-2118 (2009)
Dibyendu Bhattacharya et al.
Inorganic chemistry, 49(22), 10264-10272 (2010-10-16)
Self-assembled, chair-shaped dirhenium(I) macrocyclic compounds featuring the two different bis-chelating quinone dianions (1, L = dhnq(2-); 2, L = dhaq(2-); H(2)dhnq = 6,11-dihydroxy-5,12-naphthacenedione; H(2)dhaq = 1,4-dihydroxy-9,10-anthraquinone) that interface with two fac-Re(CO)(3) cores and a ditopic semirigid N-donor 1,4-bis(5,6-dimethylbenzimidazol-1-ylmethyl)naphthalene (L' =
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service