All Photos(2)
About This Item
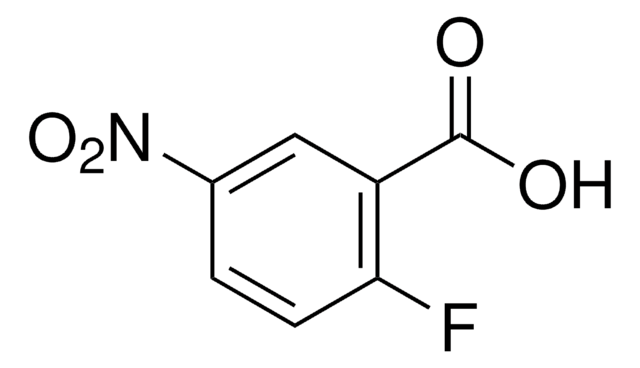
Linear Formula:
FC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
185.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
123-126 °C (lit.)
solubility
95% ethanol: soluble 50 mg/mL, clear, light yellow
functional group
carboxylic acid
fluoro
nitro
SMILES string
OC(=O)c1ccc(F)c(c1)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-5-2-1-4(7(10)11)3-6(5)9(12)13/h1-3H,(H,10,11)
InChI key
BOJWTAQWPVBIPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Fluoro-3-nitrobenzoic acid was used:
- as starting reagent in the preparation of novel benzimidazoles having antimycobacterial activity
- in preparation of series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure
- in preparation of bis(heterocyclic) skeletal precursors for the Pictet-Spengler reaction
- in solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yeong Keng Yoon et al.
Bioorganic chemistry, 49, 33-39 (2013-07-28)
Two series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure were synthesized by a four-step reaction pathway starting from 4-fluoro-3-nitrobenzoic acid as the basic compound. The structure of the novel benzimidazoles was characterized and confirmed by
Yeong Keng Yoon et al.
European journal of medicinal chemistry, 93, 614-624 (2014-07-06)
A total of 51 novel benzimidazoles were synthesized by a 4-step reaction starting from basic compound 4-fluoro-3-nitrobenzoic acid under relatively mild reaction conditions. The structure of the novel benzimidazoles was confirmed by mass spectra as well as (1)H NMR spectroscopic
Chih-Hau Chen et al.
Chemistry, an Asian journal, 6(6), 1557-1565 (2011-04-08)
A novel strategy for an unconventional Pictet-Spengler reaction has been developed for the regioselective cyclization of the imidazole ring system at the C2 position. The developed strategy was utilized to develop a diversity-oriented parallel synthesis for bis(heterocyclic) skeletal novel analogs
Gérard Klein et al.
Journal of combinatorial chemistry, 4(4), 345-351 (2002-07-09)
An efficient method for the solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones from resin-bound amino acids is described. N-acylation of the primary amine of a resin-bound amino acid with 4-fluoro-3-nitrobenzoic acid, followed by displacement of the fluoro group and reduction of the
D L Ladd et al.
Analytical biochemistry, 210(2), 258-261 (1993-05-01)
We have developed a new class of reagents (2) for the covalent attachment of polyethylene glycol to proteins. These reagents (2) are the monomethoxypolyethylene glycol esters of 4-fluoro-3-nitrobenzoic acid. The reaction of 2 with lysine epsilon-amino groups produces a chromophore
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service