All Photos(1)
About This Item
Empirical Formula (Hill Notation):
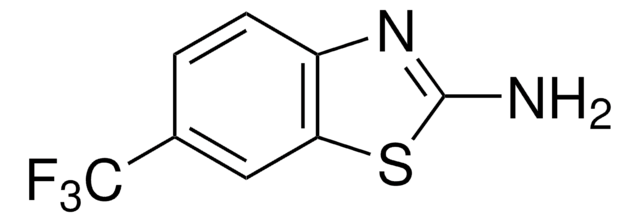
C8H8N2S
CAS Number:
Molecular Weight:
164.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
140-142 °C (lit.)
SMILES string
Cc1ccc2nc(N)sc2c1
InChI
1S/C8H8N2S/c1-5-2-3-6-7(4-5)11-8(9)10-6/h2-4H,1H3,(H2,9,10)
InChI key
DZWTXWPRWRLHIL-UHFFFAOYSA-N
Related Categories
General description
The anti-tetanus activity of 2-amino-6-methylbenzothiazole, a muscle relactant, was studied.
Application
2-Amino-6-methylbenzothiazole was used in the preparation of 2-[(6-methyl-1,3-benzothiazol-2-yl)amino]-N-[2-(substituted phenyl/furan-2-yl)-4-oxo-1,3-thiazolidin-3-yl]nicotinamides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Navin B Patel et al.
Scientia pharmaceutica, 78(4), 753-765 (2010-12-24)
The title compounds 6aâj, 2-[(6-methyl-1,3-benzothiazol-2-yl)amino]-N-[2-(substituted phenyl/furan-2-yl)-4-oxo-1,3-thiazolidin-3-yl]nicotinamides, were prepared from 2-chloropyridine-3-carboxylic acid (1) and 2-amino-6-methyl-benzothiazole (2) by known methods. All the compounds have been established by IR, (1)H NMR, (13)C NMR and elemental analyses. The in vitro antimicrobial screening of the
R A WEBSTER
British journal of pharmacology and chemotherapy, 17, 507-518 (1961-12-01)
The anti-tetanus activity of a number of phenothiazine derivatives and other centrally acting muscle relaxants, such as mephenesin, dicyclopropyl ketoxime, 2-amino-6-methylbenzothiazole and meprobamate, has been determined in rabbits with experimental local tetanus. Structure-activity relationships were obtained for the phenothiazine derivatives
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service