A97203
Azulene
99%
Synonym(s):
Bicyclo[5.3.0]decapentaene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H8
CAS Number:
Molecular Weight:
128.17
Beilstein:
969517
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
bp
242 °C (lit.)
mp
98-100 °C (lit.)
SMILES string
c1ccc2cccc2cc1
InChI
1S/C10H8/c1-2-5-9-7-4-8-10(9)6-3-1/h1-8H
InChI key
CUFNKYGDVFVPHO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mine Ince et al.
Chemical communications (Cambridge, England), 48(34), 4058-4060 (2012-03-22)
A novel supramolecular electron donor-acceptor hybrid (2·1) and an electron donor-acceptor conjugate (3), both exhibiting a remarkably shifted Q band in the NIR region of the solar spectrum, were prepared. Irradiation of the supramolecular ensemble 2·1 within the visible range
Dawei Zhao et al.
The Journal of chemical physics, 131(18), 184307-184307 (2009-11-18)
The infrared (IR) spectrum of protonated azulene (AzuH(+), C(10)H(9)(+)) has been measured in the fingerprint range (600-1800 cm(-1)) by means of IR multiple photon dissociation (IRMPD) spectroscopy in a Fourier transform ion cyclotron resonance mass spectrometer equipped with an electrospray
Hiromi Mitsuhashi et al.
European journal of pharmacology, 551(1-3), 152-155 (2006-10-19)
Oral ulcerative mucositis is a common and painful toxicity associated with chemotherapy for cancer. Current treatment for chemotherapy-induced oral mucositis is largely palliative, and no adequate treatment with conclusive evidence exists. The purpose of this study was to evaluate the
Ze-Yun Xiao et al.
Organic & biomolecular chemistry, 7(12), 2540-2547 (2009-06-09)
In this paper we report the synthesis and self-assembling behavior of new porphyrin-azulene-porphyrin and porphyrin-azulene conjugates. The porphyrin-azulene-porphyrin conjugate gelates a number of organic solvents, while the porphyrin-azulene conjugates form vesicles in a chloroform-methanol binary mixture. The structures of the
Timothy D Lash et al.
The Journal of organic chemistry, 77(5), 2368-2381 (2012-02-01)
A "2 + 2" strategy for synthesizing adj-dicarbaporphyrinoid systems has been developed. In a model study, an azulenylmethylpyrrole dialdehyde was condensed with a dipyrrylmethane in the presence of HCl, followed by oxidation with ferric chloride, to give a modest yield
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



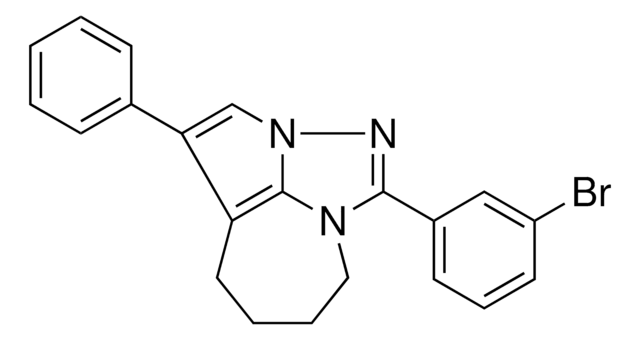
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)