855286
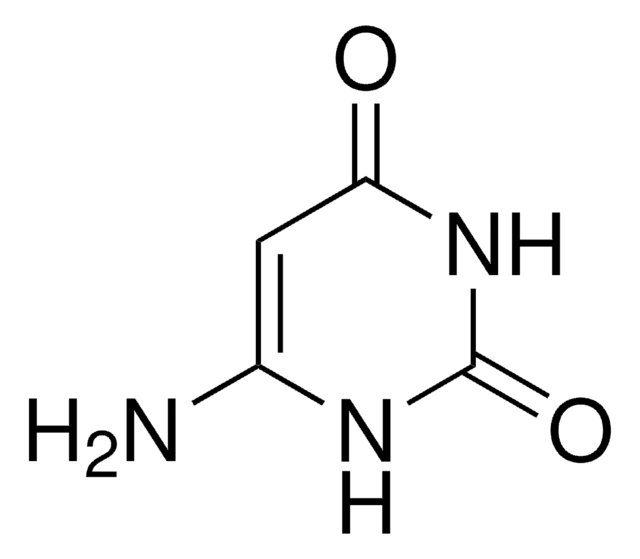
5-Aminouracil
98%
Synonym(s):
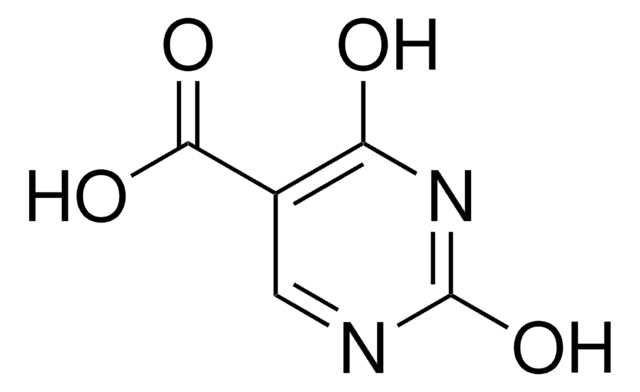
5-Amino-2,4-dihydroxypyrimidine, 5-Amino-2,4-pyrimidinediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3O2
CAS Number:
Molecular Weight:
127.10
Beilstein:
127250
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
>300 °C (lit.)
SMILES string
NC1=CNC(=O)NC1=O
InChI
1S/C4H5N3O2/c5-2-1-6-4(9)7-3(2)8/h1H,5H2,(H2,6,7,8,9)
InChI key
BISHACNKZIBDFM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Asmaa M Fahim et al.
Current computer-aided drug design, 16(4), 486-499 (2019-07-11)
In this investigation, 2-cyano-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) acetamide (3) reacts with dimethylformamide dimethyl acetal (DMF-DMA) to afford the corresponding (E)- 2-cyano-3-(dimethylamino)-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylam-ide (4) utilizing microwave irradiation. The condensation reactions of acrylamide derivative 4 with hydrazine derivatives obtain pyrazole derivatives 6a and 6b; respectively. The
Paulina Spisz et al.
International journal of molecular sciences, 21(17) (2020-09-05)
Hypoxia-a hallmark of solid tumors-dramatically impairs radiotherapy, one of the most common anticancer modalities. The adverse effect of the low-oxygen state can be eliminated by the concomitant use of a hypoxic cell radiosensitizer. In the present paper, we show that
A González-Fernández et al.
Mutation research, 149(2), 275-281 (1985-04-01)
Proliferating plant cells treated during the late S period with 5-aminouracil (AU), give the typical response that DNA-damaging agents induce, characterized by: an important mitotic delay, and a potentiation of the chromosome damage by caffeine post-treatment. The study of labelled
D Suciu
The International journal of biochemistry, 23(11), 1245-1249 (1991-01-01)
1. The results of this study have contributed to the definition of three categories of chemical inhibitors of DNA replication in mammalian cells. 2. Inhibitors of replicon cluster initiation [4-nitroquinoline-N-oxide (4-NQO), etoposide (VP-16), teniposide (VM-26), amsacrine (m-AMSA), N-methyl-N'-nitro-N-nitrozoguanidine (MNNG), cis-Pt(II)diammine
F Cortés et al.
Experimental cell research, 148(2), 503-507 (1983-10-15)
Meristematic cells of Allium cepa were treated with 5-amino-uracil (5-AU) while incorporating 5-bromodeoxyuridine (BrdU) into DNA until complete inhibition of mitosis was obtained. The pattern of BrdU substitution in interphase nuclei detected by FPG technique in the cells so treated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service