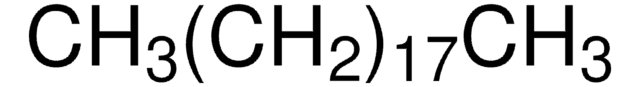76510
Pentadecane
≥98.0% (GC)
Synonym(s):
n-Pentadecane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)13CH3
CAS Number:
Molecular Weight:
212.41
Beilstein:
1698194
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
7.4 (vs air)
Quality Level
vapor pressure
1 mmHg ( 91.6 °C)
Assay
≥98.0% (GC)
form
liquid
expl. lim.
6.5 %
refractive index
n20/D 1.431 (lit.)
bp
270 °C (lit.)
mp
8-10 °C (lit.)
density
0.769 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCC
InChI
1S/C15H32/c1-3-5-7-9-11-13-15-14-12-10-8-6-4-2/h3-15H2,1-2H3
InChI key
YCOZIPAWZNQLMR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1
Supplementary Hazards
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gina M Geiselman et al.
Microbial cell factories, 19(1), 24-24 (2020-02-07)
Rhodosporidium toruloides has emerged as a promising host for the production of bioproducts from lignocellulose, in part due to its ability to grow on lignocellulosic feedstocks, tolerate growth inhibitors, and co-utilize sugars and lignin-derived monomers. Ent-kaurene derivatives have a diverse
Biosynthesis of phomactins: common intermediate phomactatriene and taxadiene.
Tetsuo Tokiwano et al.
Chemical communications (Cambridge, England), (11)(11), 1324-1325 (2004-05-22)
From 2,2'-methylenedifuran to all stereomeric pentadecane-1,3,5,7,9,11,13,15-octols.
M E Schwenter et al.
The Journal of organic chemistry, 66(23), 7869-7872 (2001-11-10)
Synthesis of homogeneous FePt nanoparticles using a nitrile ligand.
Virginie Monnier et al.
Small (Weinheim an der Bergstrasse, Germany), 4(8), 1139-1142 (2008-07-16)
Keith D Schwartz et al.
Organic letters, 13(2), 248-251 (2010-12-18)
Conjugate reduction of an enone accompanied by in situ intramolecular aldol condensation was used to construct the tetrasubstituted cyclohexane nucleus of phomactins. Subsequent relay ring-closing metathesis completed the nine-membered ansa bridge of the diterpenoid framework.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service