666548
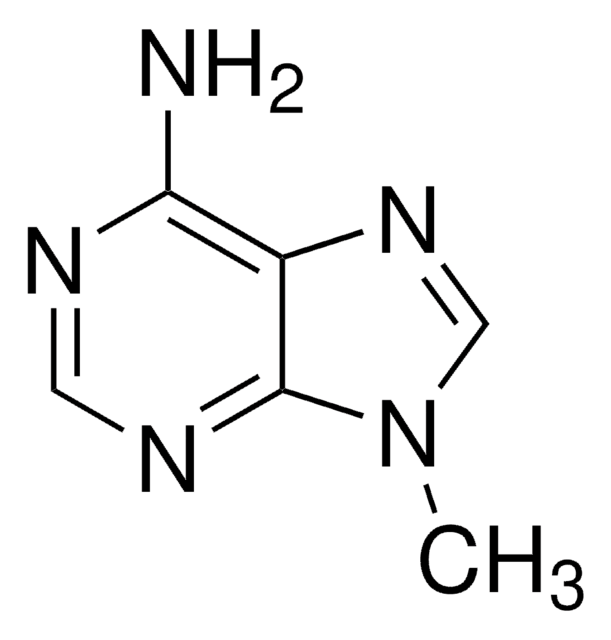
7-Methyladenine
97%
Synonym(s):
6-Amino-7-methylpurine, 7-Methyl-7H-purin-6-amine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7N5
CAS Number:
Molecular Weight:
149.15
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
346-350 °C
SMILES string
Cn1cnc2ncnc(N)c12
InChI
1S/C6H7N5/c1-11-3-10-6-4(11)5(7)8-2-9-6/h2-3H,1H3,(H2,7,8,9)
InChI key
HCGHYQLFMPXSDU-UHFFFAOYSA-N
Application
<ul>
<li><strong>Biomarkers of Cigarette Smoking and DNA Methylating Agents:</strong> Study on 7-Methyladenine highlights its role as a biomarker of DNA damage from exposure to methylating agents (Harroun et al., 2017).</li>
</ul>
<li><strong>Biomarkers of Cigarette Smoking and DNA Methylating Agents:</strong> Study on 7-Methyladenine highlights its role as a biomarker of DNA damage from exposure to methylating agents (Harroun et al., 2017).</li>
</ul>
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H G Mandel et al.
Carcinogenesis, 15(7), 1393-1398 (1994-07-01)
Earlier studies showed that urine of rats which had been injected with the methylating agent N-[3H-methyl]-N-nitrosourea contained a previously undetected metabolic product, 7-[3H-methyl]adenine. This methylpurine, undoubtedly derived from alkylation of nucleic acids followed by depurination, was not labeled when 14C-methyl-labeled
H G Mandel et al.
Carcinogenesis, 10(4), 757-762 (1989-04-01)
Relatively simple and rapid analytical procedures involving two sequential HPLC separations were developed for the isolation of methylated purines in the urine of rats administered radiolabeled methylating carcinogens. Following a dose of [3H]N-methyl-N-nitrosourea (MNU), 7-methyl-adenine (m7Gua) was detected by chromatography
Shinji Tokuda et al.
Bioscience, biotechnology, and biochemistry, 76(4), 828-830 (2012-04-10)
Adenine had a concentration-dependent relaxation action on the phenylephrine-contracted aorta ring, with an EC(50) value of 0.40±0.12 mM. This effect was also observed in the endothelium-denuded aorta. Among the adenine analogues, N-methyladenine and benzimidazole still evoked an apparent relaxation effect
H G Mandel et al.
Analytical biochemistry, 217(2), 292-297 (1994-03-01)
We have developed a procedure for isolating and quantifying 7-methyladenine from rat urine following the administration to the rat of methylating agents, such as dimethylnitrosamine. Urinary 7-methyladenine and its trideutero isomer, added as an internal standard, were precipitated with silver
B Tudek et al.
Acta biochimica Polonica, 46(3), 785-799 (2000-03-04)
The most abundant lesion formed in DNA upon modification with methylating agents 7-methylguanine, under alkaline conditions is converted into 2,6-diamino-4-hydroxy-5N-methyl-formamidopyrimidine (Fapy-7MeGua). We have previously shown that treatment of dimethylsulfate methylated DNA with NaOH creates mutagenic base derivatives leading to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service