All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5ClN2
CAS Number:
Molecular Weight:
128.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
58-62 °C
functional group
chloro
SMILES string
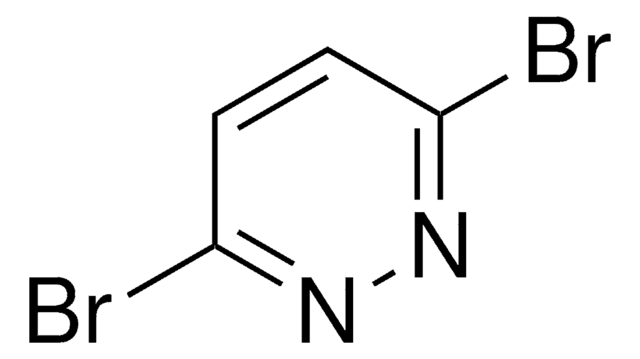
Cc1ccc(Cl)nn1
InChI
1S/C5H5ClN2/c1-4-2-3-5(6)8-7-4/h2-3H,1H3
InChI key
PRORLQAJNJMGAR-UHFFFAOYSA-N
Application
3-Chloro-6-methyl pyridazine can undergo nickel catalyzed cross coupling reaction with aromatic and heteroaromatic halides, to give the corresponding substituted aryl- and heteroaryl pyridazines. It can also be used in the synthesis of a p38MAP kinase inhibitor with therapeutic potential in the treatment of autoimmune and inflammatory diseases.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sengmany S, et al.
The Journal of Organic Chemistry, 72(15), 5631-5636 (2007)
"Development of a Practical and Scalable Synthesis of a Potent p38 Mitogen-Activated Protein Kinase Inhibitor"
Yoshida S, et al.
The Journal of Organic Chemistry, 16(11), 1818-1826 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)