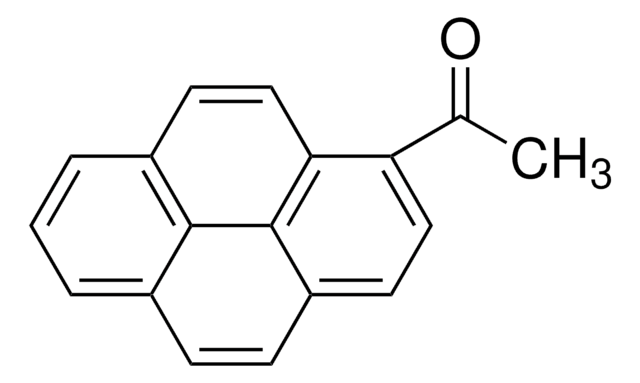
394408
1-(Bromoacetyl)pyrene
97%
Synonym(s):
2-Bromo-1-(1-pyrenyl)ethanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H11BrO
CAS Number:
Molecular Weight:
323.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
129-131 °C (lit.)
functional group
bromo
ketone
SMILES string
BrCC(=O)c1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H11BrO/c19-10-16(20)14-8-6-13-5-4-11-2-1-3-12-7-9-15(14)18(13)17(11)12/h1-9H,10H2
InChI key
KAEDEGFCOPIKKM-UHFFFAOYSA-N
Related Categories
General description
1-(Bromoacetyl)pyrene (BAP) is a pyrene derivative. It has been synthesized by reacting cupric bromide with 1-acetylpyrene. Studies suggest that the introduction of a bromoacetyl chromophoric moiety to pyrene drastically increases the photoinitiating efficiency of pyrenes.
Application
1-(Bromoacetyl)pyrene is suitable for use in the following studies:
- As an initiator in the bulk polymerization of 2-ethyl-2-oxazoline to generate pyrene labelled poly(2-ethyl-2-oxazoline) (PETOX-py).
- As a fluorophore in the generation of podand-type fluoroionophores with two pyrene moieties.
- As a fluorescent labeling agent for the determination of okadaic acid toxin by HPLC with fluorescence detection.
- As a photoremovable protecting group for carboxylic acids and amino acids.
- As a photoinitiator in the photopolymerization of styrene with methylmethacrylate.
- As a reactant in the synthesis of potentially tetradentate pyrene appended ligands.
- As a derivatizing agent of dialkyl phosphates (DAP) in the HPTLC method of quantitative determination of DAP in fruit juices.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Juraj Kronek et al.
Journal of materials science. Materials in medicine, 22(7), 1725-1734 (2011-05-24)
Poly(2-oxazolines) with varying alkyl chain lengths (e.g., methyl, ethyl, aryl) and molar masses have been tested for cell cytotoxicity in vitro. A standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used for the estimation of cell viability. Two monomers, 2-methyl-2-oxazoline and 2-ethyl-2-oxazoline
Nicola M Cox et al.
Dalton transactions (Cambridge, England : 2003), 41(5), 1568-1573 (2011-12-07)
A new method for assessing the topology of metallosupramolecular assemblies using pyrene-appended ligands is reported. Two potentially tetradentate ligands containing one (L(1)) and two (L(2)) terminal pyrene moieties were synthesised and their complexes with Cu(+) and Cd(2+) were characterised. Photophysical
S S Kelly et al.
Journal of chromatography. A, 749(1-2), 33-40 (1996-10-18)
The rare diarrhetic shellfish toxin, dinophysistoxin-2 (DTX-2), was isolated from the digestive glands of mussels (Mytilus edulis). This was achieved by chromatography on silica and Sephadex LH-20 followed by reversed-phase solid phase extraction and semi-preparative high-performance liquid chromatography (HPLC) with
Copolymerization of n-butylacrylate with styrene by a novel photoinitiator, 1-(bromoacetyl) pyrene.
Mishra A and Daswal S
Journal of Applied Polymer Science, 102.4, 3233-3239 (2006)
J C González et al.
Journal of chromatography. A, 793(1), 63-70 (1998-02-20)
Okadaic acid (OA) and dinophysistoxin-2, two of the main diarrhetic shellfish toxins, can be determined by high-performance liquid chromatography coupled to fluorimetry as pyrenacyl esters. Toxin fluorescent derivatives were obtained after quantitative derivatization with 1-bromoacetylpyrene in acetonitrile. An efficient improvement
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service