HPA012628
Anti-GALNT1 antibody produced in rabbit
Prestige Antibodies® Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution
Synonyme(s) :
Anti-GalNAc-T1, Anti-Polypeptide GalNAc transferase 1, Anti-Polypeptide N-acetylgalactosaminyltransferase 1, Anti-Protein-UDP acetylgalactosaminyltransferase 1, Anti-UDP- GalNAc:polypeptide N-acetylgalactosaminyltransferase 1, Anti-pp-GaNTase 1
About This Item
Produits recommandés
Source biologique
rabbit
Conjugué
unconjugated
Forme d'anticorps
affinity isolated antibody
Type de produit anticorps
primary antibodies
Clone
polyclonal
Gamme de produits
Prestige Antibodies® Powered by Atlas Antibodies
Forme
buffered aqueous glycerol solution
Espèces réactives
human
Technique(s)
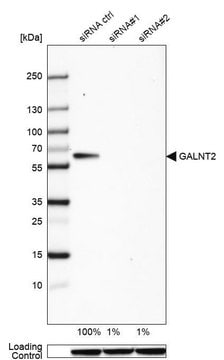
immunoblotting: 0.04-0.4 μg/mL
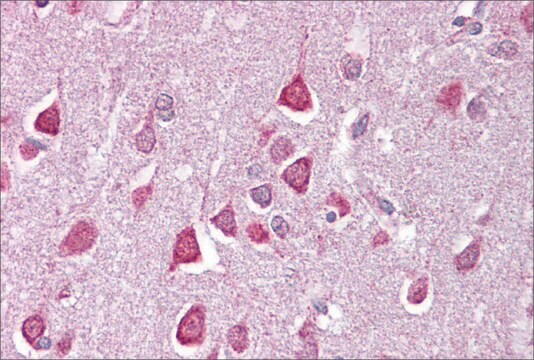
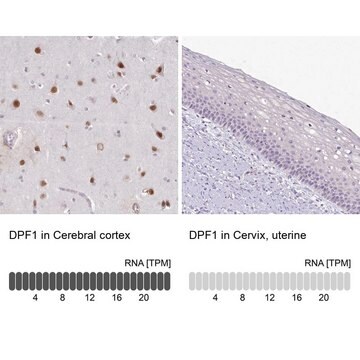
immunohistochemistry: 1:50-1:200
Séquence immunogène
KERGLPAGDVLEPVQKPHEGPGEMGKPVVIPKEDQEKMKEMFKINQFNLMASEMIALNRSLPD
Numéro d'accès UniProt
Conditions d'expédition
wet ice
Température de stockage
−20°C
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
human ... GALNT1(2589)
Description générale
Immunogène
Application
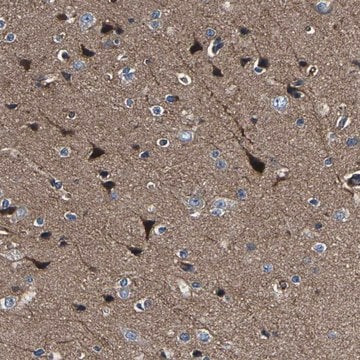
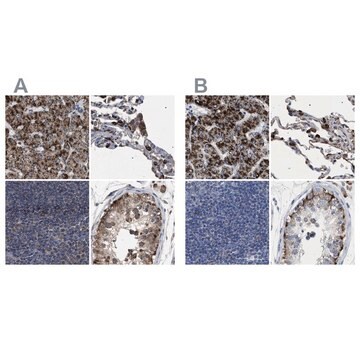
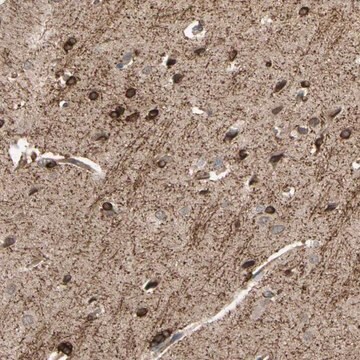
The Human Protein Atlas project can be subdivided into three efforts: Human Tissue Atlas, Cancer Atlas, and Human Cell Atlas. The antibodies that have been generated in support of the Tissue and Cancer Atlas projects have been tested by immunohistochemistry against hundreds of normal and disease tissues and through the recent efforts of the Human Cell Atlas project, many have been characterized by immunofluorescence to map the human proteome not only at the tissue level but now at the subcellular level. These images and the collection of this vast data set can be viewed on the Human Protein Atlas (HPA) site by clicking on the Image Gallery link. We also provide Prestige Antibodies® protocols and other useful information.
Actions biochimiques/physiologiques
Caractéristiques et avantages
Every Prestige Antibody is tested in the following ways:
- IHC tissue array of 44 normal human tissues and 20 of the most common cancer type tissues.
- Protein array of 364 human recombinant protein fragments.
Liaison
Forme physique
Informations légales
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique