MAB1012
Anti-Alkaline Phosphatase Antibody, E. coli, bacterial only
ascites fluid, Chemicon®
Synonyme(s) :
AP, Alk. Phos.
About This Item
Produits recommandés
Source biologique
mouse
Niveau de qualité
Forme d'anticorps
ascites fluid
Type de produit anticorps
primary antibodies
Clone
monoclonal
Espèces réactives
E. coli
Fabricant/nom de marque
Chemicon®
Technique(s)
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
Isotype
IgG2a
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
Escherichia coli ... PhoA(945041)
Spécificité
Immunogène
Application
Immunocytochemistry: reacts with E.coli. AP fusion protein targets in acetone fixed cell preparations. 1:4000, other fixatives or conditions untested.
ASSAY:
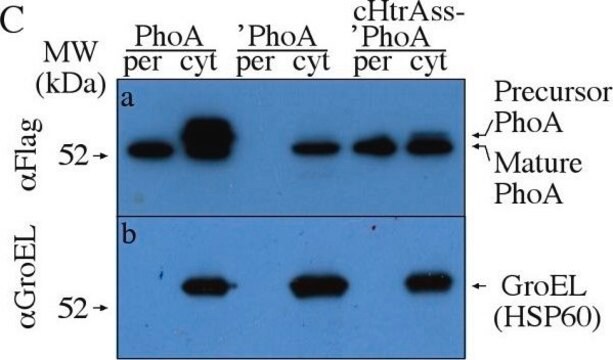
Preparation of E. coli TnphoA transformants: E. coli strain CC118 was transformed with plasmid pGEM-3Z containing TnphoA insertional mutations in the p101 gene of Mycoplasma hyorhinis, which encodes a protein with a typical N-terminal prokaryotic single peptide (Yogev et al. 1991).
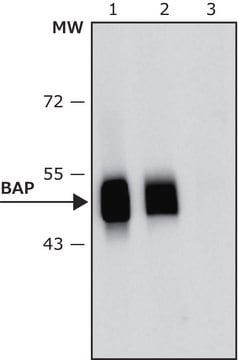
Identification of fusion protein with MAB1012 transformants: Transformants are grown in 2XYT medium to OD600=0.6. Cells were centrifuged 3 minutes at 10,000 x g, suspended in SDS-PAGE sample buffer, heated at 100°C for 5 minutes, frozen and thawed and centrifuged as above at room temperature to remove insoluble material. The sample is applied at 9% to a SDS-PAGE gel, and Western immunoblot is performed as described (Yogev et al. 1991).
Immunoprecipitation: 5μL of antibody per 500μL of lysate in RIPA or 0.5% triton X-100 solutions.
Optimal working dilutions must be determined by end user.
Forme physique
Informations légales
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique