All Photos(1)
About This Item
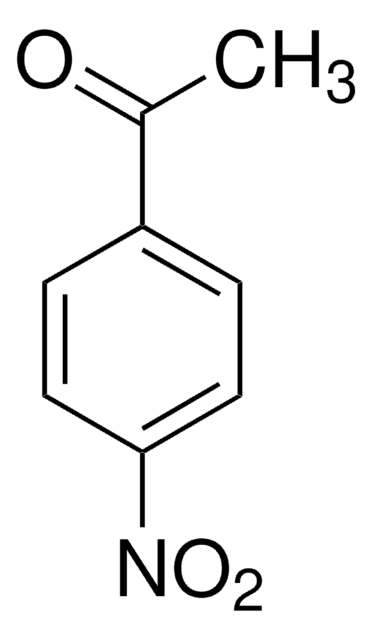
Linear Formula:
F2C6H3COCH3
CAS Number:
Molecular Weight:
156.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
34-38 °C (lit.)
functional group
fluoro
ketone
SMILES string
CC(=O)c1cc(F)cc(F)c1
InChI
1S/C8H6F2O/c1-5(11)6-2-7(9)4-8(10)3-6/h2-4H,1H3
InChI key
OXJLDNSPGPBDCP-UHFFFAOYSA-N
General description
3′,5′-Difluoroacetophenone, also known as 1-(3,5-difluorophenyl)ethanone, is a fluorinated acetophenone.
Application
3′,5′-Difluoroacetophenone (3,5-Difluoroacetophenone) may be used to synthesize:
- (E)-3-(4-(1,5,9-trithia-13-azacyclohexadecan-13-yl)-phenyl)-1-(3,5-difluorophenyl)prop-2-en-1-one
- 1,3,5-triarylpyrazoline fluorophores containing a 16-membered thiazacrown ligand
- (±)-fluorinated-1-(3-morpholin-4-yl-phenyl)ethylamine
- (E)-1-(3,5-difluorophenyl)-3-(2,4-dimethoxyphenyl) prop-2-en-1-one
- 1-(3,5-difluorophenyl)-4,4,4-trifluorobutane-1,3-dione
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
180.0 °F - closed cup
Flash Point(C)
82.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
1-(3, 5-Difluorophenyl)-4, 4, 4-trifluorobutane-1, 3-dione.
Manoj Kumar KE, et al.
Acta Crystallographica Section E, Structure Reports Online, 69(11), o1705-o1705 (2013)
(E)-1-(3, 5-Difluorophenyl)-3-(2, 4-dimethoxyphenyl) prop-2-en-1-one.
Huang T, et al.
Acta Crystallographica Section E, Structure Reports Online, 66(10), o2518-o2518 (2010)
Manjusha Verma et al.
Organic & biomolecular chemistry, 8(2), 363-370 (2010-01-13)
We have prepared and characterized a Cu(i)-responsive fluorescent probe, constructed using a large tetradentate, 16-membered thiazacrown ligand ([16]aneNS(3)) and 1,3,5-triaryl-substituted pyrazoline fluorophores. The fluorescence contrast ratio upon analyte binding, which is mainly governed by changes of the photoinduced electron transfer
Yong-Jin Wu et al.
Bioorganic & medicinal chemistry letters, 13(10), 1725-1728 (2003-05-06)
The synthesis of four (+/-)-fluorinated 1-(3-morpholin-4-yl-phenyl)-ethylamines and an enantioselective approach to these amines through reductive amination are described.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service