534579
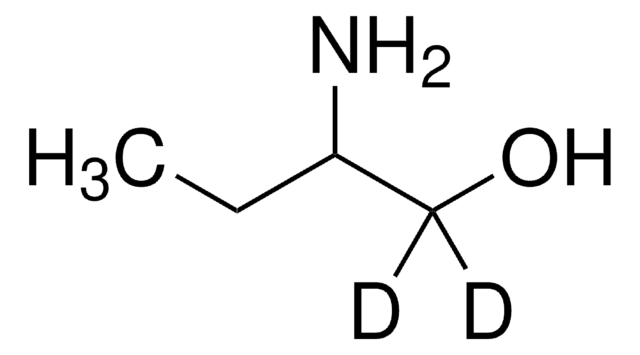
(R)-(−)-2-Amino-1-pentanol
97%
Synonym(s):
D-Norvalinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)2CH(NH2)CH2OH
CAS Number:
Molecular Weight:
103.16
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D −17°, c = 1 in chloroform
mp
44-48 °C (lit.)
SMILES string
CCC[C@@H](N)CO
InChI
1S/C5H13NO/c1-2-3-5(6)4-7/h5,7H,2-4,6H2,1H3/t5-/m1/s1
InChI key
ULAXUFGARZZKTK-RXMQYKEDSA-N
Application
(R)-(-)-2-Amino-1-pentanol can be used as a chiral building block to prepare:
- A key intermediate, (R)-N-(p-toluenesulfonyl)-2-propylaziridine, which is utilized in the total synthesis of (R)-1-(benzofuran-2-yl)-2-propylaminopentane.
- (R)-N-benzyloxycarbonyl-aminoaldehydes as potential substrates for dihydroxyacetone phosphate (DHAP)-dependent aldolases.
- (R)-2-((2-Aminoquinazolin-4-yl)amino)pentan-1-ol as a potent dual toll-like receptor and modulator.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dihydroxyacetone phosphate aldolase catalyzed synthesis of structurally diverse polyhydroxylated pyrrolidine derivatives and evaluation of their glycosidase inhibitory properties
Calveras J, et al.
Chemistry?A European Journal , 15(30), 7310-7328 (2009)
2, 4-diaminoquinazolines as dual toll-like receptor (TLR) 7/8 modulators for the treatment of hepatitis B virus
Embrechts W, et al.
Journal of Medicinal Chemistry, 61(14), 6236-6246 (2018)
Enantioselective synthesis and absolute configuration of (-)-1-(benzofuran-2-yl)-2-propylaminopentane,((-)-BPAP), a highly potent and selective catecholaminergic activity enhancer
Oka T, et al.
Bioorganic & Medicinal Chemistry, 9(5), 1213-1219 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service