All Photos(1)
About This Item
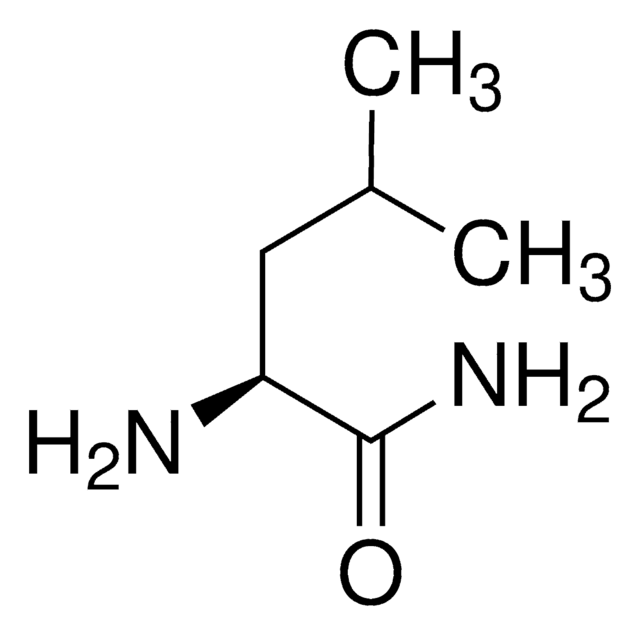
Linear Formula:
(CH3)2CHCH2CH(NH2)CONH2·HCl
CAS Number:
Molecular Weight:
166.65
Beilstein:
4237021
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D +10°, c = 5 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
254-256 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl.CC(C)C[C@H](N)C(N)=O
InChI
1S/C6H14N2O.ClH/c1-4(2)3-5(7)6(8)9;/h4-5H,3,7H2,1-2H3,(H2,8,9);1H/t5-;/m0./s1
InChI key
VSPSRRBIXFUMOU-JEDNCBNOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide: lack of generality.
R Mehvar et al.
Journal of chromatography, 431(1), 228-230 (1988-09-23)
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide.
H Spahn
Journal of chromatography, 423, 334-339 (1987-12-25)
B Grinde et al.
Acta biologica et medica Germanica, 40(10-11), 1603-1612 (1981-01-01)
An amino acid mixture, specially designed to improve the protein balance in isolated hepatocytes, inhibited lysosomal (propylamine-sensitive) degradation of endogenous proteins by 80-90%. The amino acids had no effect on the degradation of the endocytosed protein asialofetuin, the conclusion being
[Marked dissociation of leucine aminopeptidase activities by the use of 2 different substrates--application of the methods to lymphatic diseases].
Y Hirasawa et al.
Nihon Ketsueki Gakkai zasshi : journal of Japan Haematological Society, 45(5), 907-912 (1982-09-01)
Y L Wang et al.
Solid state nuclear magnetic resonance, 14(1), 19-32 (1999-07-17)
Proton NMR relaxation time measurements were carried out on solid tyrosine derivatives: acetyl-L-tyrosine ethyl ester (Ac-Tyroet), N-carbobenzyloxy-L-tyrosine ethyl ester (CBZ-Tyroet), N-trifluoroacetyl-L-tyrosine ethyl ester (TFAc-Tyroet) and their mixtures with L-leucinamide. It was found that spin-lattice relaxation was driven mainly by methyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service