Validated Human Cell Types for Millicell® DCI Digital Cell Imager
The Millicell® DCI Digital Cell Imager provides quick, objective determination of common cell culture parameters for more consistent cell cultures, enabling more efficient execution of the repetitive, daily techniques associated with cell passaging. Monitor an extensive range of adherent cell cultures including 3D cultures and tricky or hard-to-grow cells. Our R&D team has been working to validate the Millicell® DCI across a broad range of cell types, including human-derived cell lines to ensure optimal instrument performance. Select a cell type below to view the data.
- Catalog number: 85090402
- Source: Human skin (female), squamous carcinoma
- Cell type/morphology: Adherent, epithelial
- Application: To study p38 MAPK-induced apoptosis, to study melanoma-specific CD44 receptor, to study the effect of anti-cancer medical plants
- Biosafety level: 2
- Handling: Easy
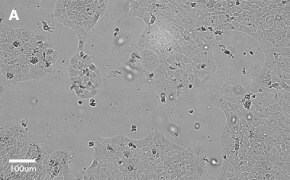
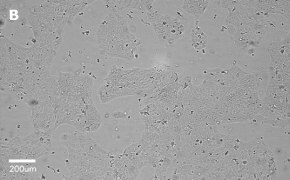
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).

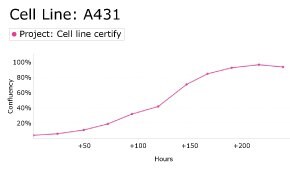
8. Growth Curve
9. Cell culture media:
EMEM (EBSS) + 2 mM L-Glutamine (G7513) + 1% Non-Essential Amino Acids (NEAA) + 10% FBS (F2442).
Split sub-confluent cultures (70-80%) 1:3 to 1:6, i.e., seeding at 2-4 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
- Catalog number: 86012804
- Source: Human, lung (carcinoma)
- Cell type/morphology: Adherent, epithelial
- Application: To study the role of BChTT enzyme in cancer inhibition, to study the effects of insulin and IGF1, to study alveolar type II differentiation
- Biosafety level: 2
- Handling: Easy
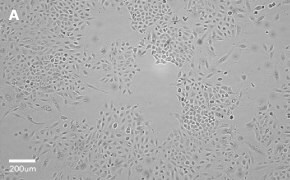
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
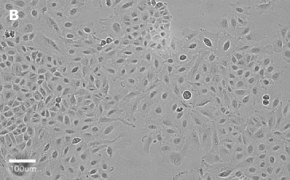
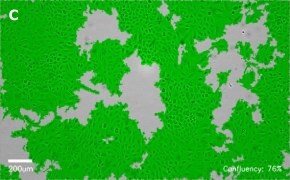
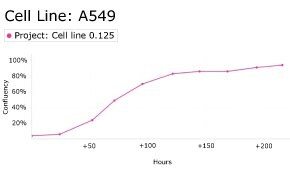
8. Growth Curve
- Catalog number: SCC430
- Source: Human bone marrow
- Cell type/morphology: Suspension cell line
- Application: Plasma cell myeloma/amyloidosis
- Biosafety level: 2
- Handling: Medium
- Images: Brightfield image on Millicell® DCI (A) and flow cytometry histogram showing expression of CD44 in yellow and unstained cells in gray (B)


- Genetic profile:
- Cell culture media:
Cells were thawed in ALMC-1 recovery medium and expanded in ALMC-1 Expansion Medium comprised of IMDM medium (I6529) with 10% FBS (ES-009-M), 2 mM L-Glutamine (TMS-002-C), 2 ng/mL IL-6 (GF338) and 1X penicillin/streptomycin (TMS-AB2-C, optional). - Protocols: Cell culture protocols are available in product datasheet.
- Scepter™ histogram: Cells were counted on a Scepter™ 3.0 automated cell counter.

- Catalog number: 86010202
- Source: Human colon (Caucasian colon adenocarcinoma)
- Cell type/morphology: Adherent, epithelial
- Application: To study if components of the sigma B regulon in Listeria monocytogenes contribute to cell invasion, for functional analysis of bacteriocin divercin AS7, to study the intracellular invasion by Listeria ivanovii
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B), and the 10X image with confluency mask (C).



- Growth curve:

- Cell culture media:
EMEM (EBSS) (M2279) + 2 mM Glutamine (G7513) + 1% Non-Essential Amino Acids (NEAA) (M7145) + 10% Fetal Bovine Serum (FBS) (ES-009-M).
Split sub-confluent cultures (70-80%) 1:3 to 1:6, i.e. seeding at 2-4 x 10,000 cells/cm2 using 0.25% Trypsin or Trypsin/EDTA; 5% CO2; 37°C. During routine subculture, the cells should always be subcultured before they achieve confluence. Cells may show the appearance of circular vacuoles in the cytoplasm. These may increase in frequency as the culture density increases to confluence. To reduce their frequency, media change confluent cultures after 2-3 days if not subcultured. Cells can clump if not separated into a single-cell suspension when split. - Protocols: Cell culture protocols are available in the product datasheet.
- Scepter™ histogram: Cells were counted on a Scepter™ 3.0 automated cell counter.

- Catalog number: 91091005
- Source: Human colon carcinoma
- Cell type/morphology: Adherent, epithelial-like
- Application: Tumorigenicity studies; to study the importance of cyclin D1 for the activity of lithocholic acid hydroxyamide (LCAHA); to study the uptake of fluorescently-labelled siRNA; to study the effect of polyamine transport system in the selective uptake of Quilamine HQ1-44, an iron chelator
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
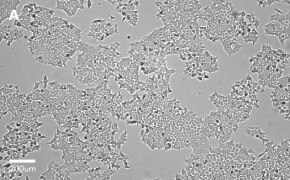
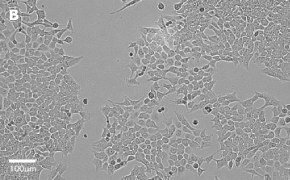
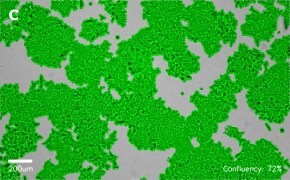
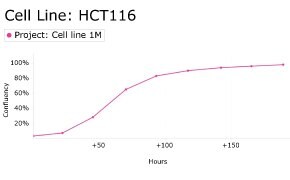
8. Growth Curve
9. Cell culture media:
McCoy’s 5a medium (D6546) + 2 mM L-Glutamine (G7513) + 10% FBS (F2442).
Split sub-confluent cultures (70-80%) 1:3 to 1:10, i.e., seeding at 1-3 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
- Catalog number: 12022001
- Source: Human, fetal kidney cell
- Cell type/morphology: Adherent, small slightly rounded cells growing in a dispersed manner
- Application: Lentiviral particle production, expression of eukaryotic proteins, siRNA transfection, HIV transfection for pseudoviruses production
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
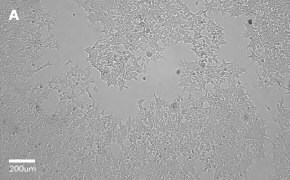
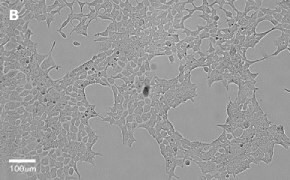

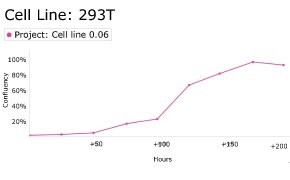
8. Growth Curve
9. Cell culture media:
DMEM-Hi-glucose medium (D6546) + 2 mM L-Glutamine (G7513) + 10% FBS (F2442).
Split sub-confluent cultures (70-80%) 1:4 to 1:10, i.e., seeding at 2-4 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
11. Scepter™ histogram: Cells were diluted 1:32 and counted using a Scepter™ 3.0 automated cell counter using 60 µm sensor.
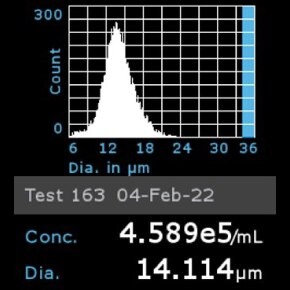
- Catalog number: 93021013
- Source: Human cervix, epitheloid cervix carcinoma
- Cell type/morphology: Adherent, epithelial
- Application: To study Zika virus (ZIKV) infection; Virus studies; to study the cytotoxicity of extracts, fruits and jams from Sorbus sps
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
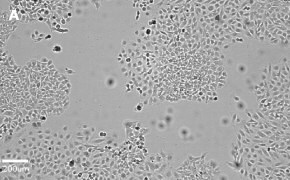
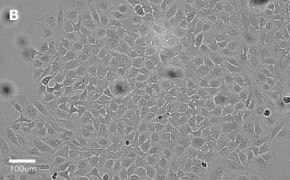
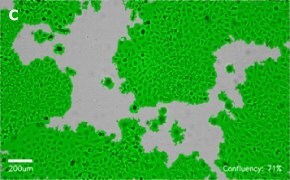
8. Growth Curve
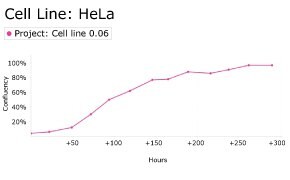
9. Cell culture media:
EMEM (EBSS) + 2 mM L-Glutamine (G7513) + 1% Non-Essential Amino Acids (NEAA) + 10% FBS/FCS (F2442).
Split sub-confluent cultures (70-80%) 1:3 to 1:6, i.e., seeding at 1-3 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
- Catalog number: 91072201
- Source: Human colon
- Cell type/morphology: Adherent, epithelial
- Application: HT29 Cell Line has been used to determine viral titers of human parechovirus. Study the ability of Bifidobacterium and Lactobacillus strains to counteract the toxic effect of C. difficile LMG21717, Tumorigenicity studies
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B), and the 10X image with confluency mask (C).



- Growth curve:

- Cell culture media:
McCoy′s5A (M8403) + 2 mM Glutamine (G7513) + 10% Fetal Bovine Serum FBS/FCS (F2442).
Split sub-confluent cultures (70-80%) 1:3 to 1:10, i.e. seeding at 1-3 x 10,000 cells/cm2 using 0.25% Trypsin; 5% CO2; 37°C. - Protocols: Cell culture protocols are available in the product datasheet.
- Scepter™ histogram: Cells were counted on a Scepter™ 3.0 automated cell counter.

- Catalog number: 01042712
- Source: Human liver
- Cell type/morphology: Adherent, epithelial
- Application: Study of intracellular traffic of delta virus antigens and RNA, viral RNA replication and early virus assembly events, and interaction between HDV and HBV
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
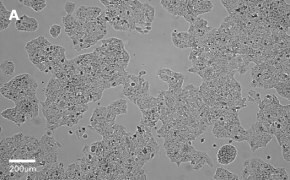
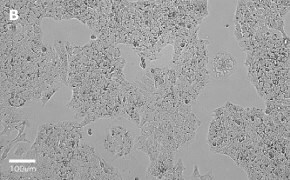
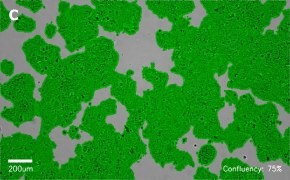
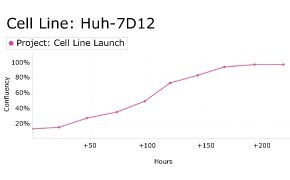
8. Growth Curve
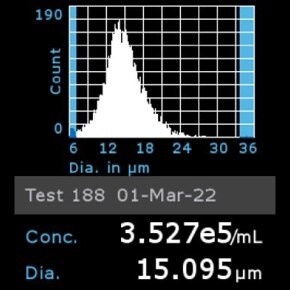
9. Cell culture media:
DMEM-Hi-glucose medium (D6546) + 2 mM L-Glutamine (G7513) + 10% FBS (F2442).
Split sub-confluent cultures (70-80%) 1:3 to 1:6, i.e., seeding at 3-5 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
11. Scepter™ histogram: Cells were diluted 1:32 and counted using a Scepter™ 3.0 automated cell counter using 60 µm sensor.
- Catalog number: 85020204
- Source: Human lung (fetal)
- Cell type/morphology: Adherent, fibroblast
- Application: Virus studies, study the cytotoxicity of hydroxyapatite/gold /arginine (HAp/Au/arginine) nanoparticles.
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B), and the 10X image with confluency mask (C).



- Growth curve:

- Cell culture media:
EMEM (EBSS) (M2279) + 2 mM Glutamine (TMS-002-C) + 1% Non-Essential Amino Acids (NEAA) (TMS-001-C) + 10% Fetal Bovine Serum (FBS) (ES-009-M).
Split sub-confluent cultures (70-80%) 1:3 to 1:6; i.e. seeding at 2-3 x 10,000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
- Protocols: Cell culture protocols are available in the product datasheet.
- Scepter™ histogram: Cells were counted on a Scepter™ 3.0 automated cell counter.

- Catalog number: SCC613
- Source: Human liver
- Cell type/morphology: Hepatic stellate cells, adherent
- Application: Model of human hepatic fibrosis
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A) and 10X image with confluency mask (B)


- Genetic profile:

- Catalog number: 86012803
- Source: Human, breast (adenocarcinoma)
- Cell type/morphology: Adherent, epithelial-like
- Application: Tumorigenicity and virus studies: type B and C viruses
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
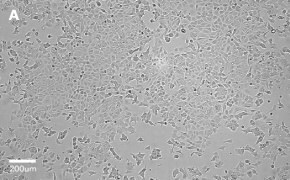
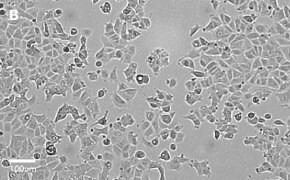

8. Growth Curve
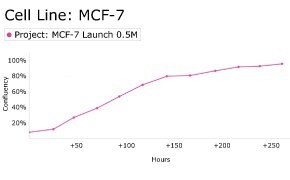
9. Cell culture media:
EMEM (EBSS) + 2 mM L-Glutamine (G7513) + 1% Non-Essential Amino Acids (NEAA) + 10% FBS/FCS (F2442).
Split sub-confluent cultures (70-80%) 1:2 to 1:6, i.e., seeding at 2-4 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
- Catalog number: SCC276
- Source: Human breast cancer
- Cell type/morphology: Adherent, epithelial
- Application: Cellular model for estrogen-responsive metastatic breast cancer
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A) and 10X image with confluency mask (B)


- Genetic profile:

- Catalog number: 86051601
- Source: Human osteosarcoma
- Cell type/morphology: Adherent, fibroblast
- Application: Interferon-induction studies, to investigate the cytotoxicity of acrylic bone cement extracts on MG-63 cells, to fabricate three-dimensional (3-D) niches and study the cell viability, adhesion, and proliferation of MG-63 cells
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B), and the 10X image with confluency mask (C).



- Growth curve:

- Cell culture media:
EMEM (EBSS) (M2279) + 2 mM Glutamine (TMS-002-C) + 1% Non-Essential Amino Acids (NEAA) (TMS-001-C) + 10% Fetal Bovine Serum (FBS) (ES-009-M).
Split sub-confluent cultures (70-80%) 1:3 to 1:6, i.e. seeding at 2-4 x 10,000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C. - Protocols: Cell culture protocols are available in the product datasheet.
- Scepter™ histogram: Cells were counted on a Scepter™ 3.0 automated cell counter.

- Catalog number: 84100401
- Source: Human, fetal lung cell
- Cell type/morphology: Adherent, fibroblast-like fetal lung cell
- Application: Transformation and virus studies: SV40
- Biosafety level: 2
- Handling: Easy
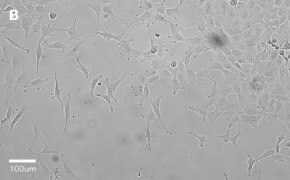
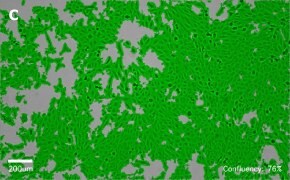
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
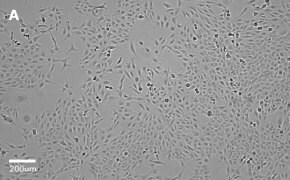
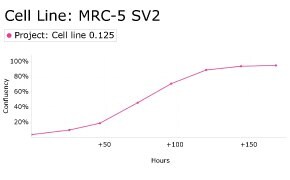
8. Growth Curve
9. Cell culture media:
EMEM (EBSS) + 2 mM L-Glutamine (G7513) + 1% Non-Essential Amino Acids (NEAA) + 10% FBS (F2442).
Split sub-confluent cultures (70-80%) 1:5 to 1:10, i.e., seeding at 1-2 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
10. Protocols: Cell culture protocols are available in product datasheet.
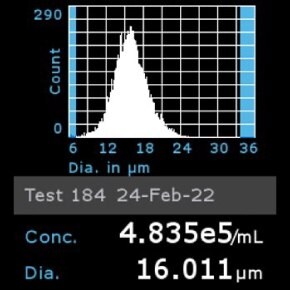
11. Scepter™ histogram: Cells were diluted 1:8 and counted using a Scepter™ 3.0 automated cell counter using 60 µm sensor.
- Catalog number: SCC468
- Source: Human bladder carcinoma
- Cell type/morphology: Adherent, epithelial
- Application: To study metastatic bladder cancer
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A) and 10X image with confluency mask (B)


- Genetic profile:

- Catalog number: 90020107
- Source: Human, Female Caucasian, fetal lung cell
- Cell type/morphology: Adherent, fibroblast-like fetal lung cell
- Application: Virus studies (rhinoviruses), vaccine production, study cytotoxicity of acrylic based resin compounds and silica nanoparticles
- Biosafety level: 2
- Handling: Easy
- Images: Brightfield image on Millicell® DCI at 10X (A), 20X (B) and the 10X image with confluency mask (C).
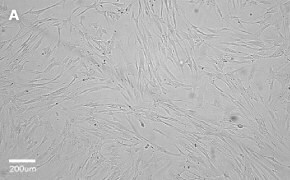
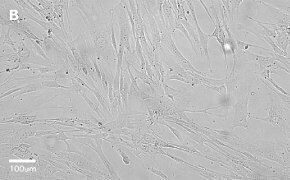

8. Growth Curve
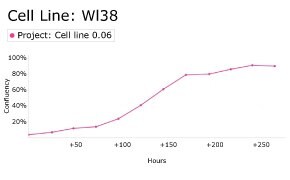
9. Cell culture media:
EMEM (EBSS) + 2 mM L-Glutamine (G7513) + 1% Non-Essential Amino Acids (NEAA) + 15% FBS (F2442).
Split sub-confluent cultures (70-80%) 1:3 to 1:6, i.e., seeding at 2-4 x 10,0000 cells/cm2 using 0.25% trypsin or trypsin/EDTA; 5% CO2; 37 °C.
Finite lifespan, 50 ± 10 population doublings.
10. Protocols: Cell culture protocols are available in product datasheet.
11. Scepter™ histogram: Cells were diluted 1:8 and counted using a Scepter™ 3.0 automated cell counter using 60 µm sensor.
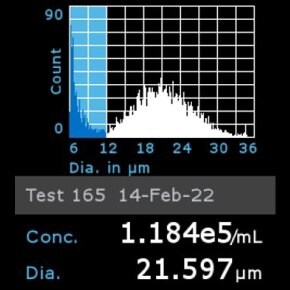
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?