Obtaining Ultrapure Water for Sensitive PFAS Analysis by LC-MS
Estelle Riche PhD, Patricia Renard, Jean-Christophe Royer
Lab Water Solutions, MilliporeSigma, Guyancourt, France

Section Overview
- PFAS, the “forever chemicals”
- PFAS testing requires ultrapure water free of detectable PFAS
- Study: Is water from a Milli-Q® IQ 7000 purification system suitable for PFAS testing?
- Components of an optimal water purification system for PFAS analysis
- Conclusion: Ultrapure water suitable for sensitive PFAS analyses
- Related Products & Ordering Information
Ultrapure water from a Milli-Q® water purification system was tested for PFAS according to the draft EPA 1633 method. None of the 40 PFAS compounds tested were detected in the ultrapure water produced by a Milli-Q® IQ 7000 system fitted with a LC-Pak® polisher at the point-of-dispense, making it suitable for the most sensitive PFAS analyses by LC-MS/MS.
PFAS, the “forever chemicals”
What are PFAS?
Per- and polyfluoroalkyl substances (PFAS) have been used worldwide in industry since the 1940s. PFAS are man-made chemicals, made of a carbon chain of variable length with very stable carbon-fluorine bonds (Figure 1). Their unique water-, grease- and stain-resistant properties make them ideal for many products, such as food packaging, nonstick cookware, water repellent and stain resistant fabrics, cosmetics, and firefighting foams.1 They are also critical in the manufacture of many products important for modern life, including medical technologies, semiconductors, batteries, phones, automobiles and airplanes.
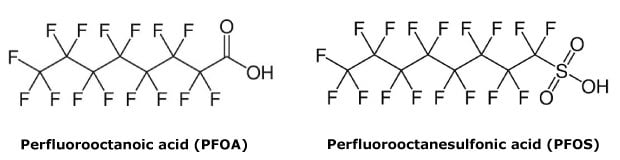
Figure 1.Chemical structures of two common PFAS compounds: PFOA and PFOS.
Environmental and health concerns of PFAS
Unfortunately, the characteristics that make these compounds useful also lead to pervasive contamination in the environment. PFAS are sometimes called “forever chemicals” since their strong carbon-fluorine bonds allow them to resist degradation. The more carbon atoms they contain, the more persistent they are in the environment. Two of the best-known PFAS, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), have the highest levels of environmental persistence.2
Research suggests that high levels of certain PFAS may have an impact on human health and lead to increased cholesterol levels, decreased vaccine response in children, increased risk of high blood pressure or pre-eclampsia in pregnant women, and an increased risk of kidney or testicular cancer.3
PFAS Regulations
PFAS are a worldwide concern. Many countries have already phased out, or are planning to phase out or regulate, some of these compounds. The Stockholm Convention, an international agreement on specific Persistent Organic Pollutants (POPs), regulates several PFAS at a global level. In Europe, PFOS is restricted under the EU’s POPs regulation, and several perfluorinated carboxylic acids, salts and precursors are restricted under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation.4 In the US, the Environmental Protection Agency (EPA) issued a National Primary Drinking Water Regulation (NPDWR) for several PFAS substances in early 2024.5
PFAS testing requires ultrapure water free of detectable PFAS
LC-MS/MS (liquid chromatography with tandem mass spectrometry) is the most used method for sensitive PFAS analyses. One of the challenges of these analyses resides in the fact that many laboratory reagents and equipment may leach some PFAS, and potentially interfere with results. Background contamination can originate from LC components containing fluoropolymers (e.g., PTFE) or from mobile phase solvents. Using a specific PFAS delay column can prevent background PFAS contamination from interfering with the sample results (Figure 2).
Reagent water plays a critical role in trace analyses of PFAS: it is used in many steps of the analytical workflow, from rinsing glassware and SPE cartridges to preparing mobile phases, calibration standards and blanks. Even if using a delay (or trap) column can alleviate some background issues, water is also used in steps where the delay column does not help (e.g., standard and sample preparation), so it needs to be free of detectable PFAS. To obtain reliable and accurate analyses of PFAS, it is important to use ultrapure water free of detectable levels of PFAS.
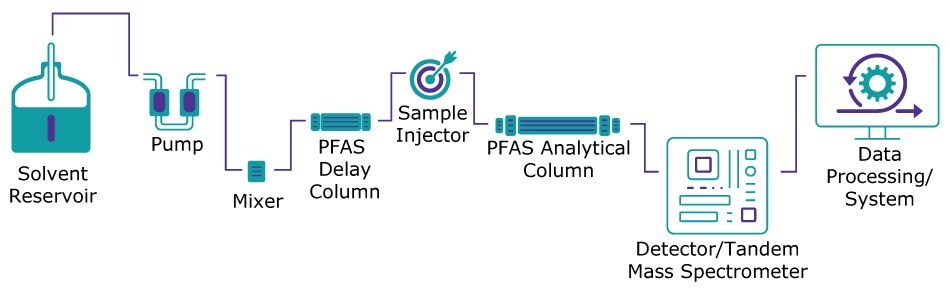
Figure 2.LC-MS/MS instrument including a PFAS delay column.
Study: Is water from a Milli-Q® IQ 7000 purification system suitable for PFAS testing?
Experimental set-up
PFAS compounds are ubiquitous in labs and may be present in many plastics and instrumentation components. To ascertain that Milli-Q® water systems (1) can remove traces of PFAS from tap water and (2) do not release PFAS into purified water, two water types were tested:
- Tap water, obtained from our laboratories in Burlington, MA, USA where the tests were conducted.
- Ultrapure water produced by a Milli-Q® IQ 7000 system with a polisher specifically designed for sensitive organic analyses (LC-Pak® polisher) placed at the point of water delivery. The ultrapure water system was fed by pure water from a high-throughput Milli-Q® HX 7 series system (Figure 3).
Samples were analyzed by a third-party laboratory following the EPA 1633 draft method.6
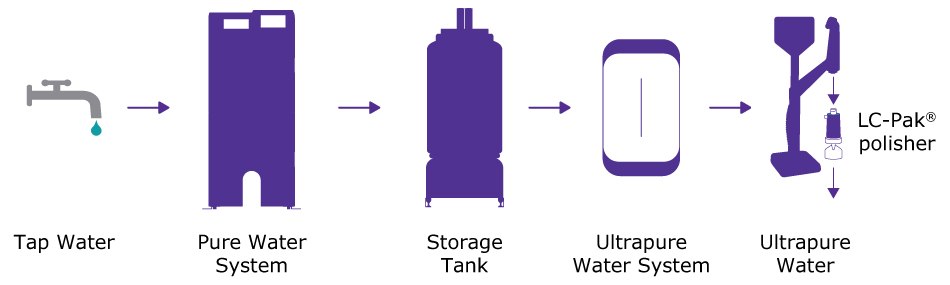
Figure 3.Schematic depicting the water purification system set-up delivering the ultrapure water tested. Tap water fed a high-throughput water purification system (Milli-Q® HX 7 series), which delivered Elix® pure water through a distribution loop to an ultrapure water system (Milli-Q® IQ 7000) fitted with a reverse-phase C18 silica-based cartridge (LC-Pak® polisher) at the point of delivery.
A few PFAS compounds could be detected in the tap water of the laboratory (PFHxA, PFHpA, PFOA, PFNA), albeit at very low levels (Table 1). However, no detectable levels of PFAS were found in the ultrapure water delivered by the Milli-Q® IQ 7000 system fitted with an LC-Pak® polisher. These results show that the water purification systems tested removed traces of PFAS present in tap water and delivered highly purified water absent of detectable amounts of PFAS.
Results
Components of an optimal water purification system for PFAS analysis
It has been shown that most PFAS molecules are large enough to be retained by reverse osmosis (RO) membranes and are also efficiently retained by activated carbon.8 In addition, since PFAS are charged molecules, they can be removed by ion-exchange resins and by electrodeionization (EDI). A Milli-Q® water purification system that combines RO, activated carbon and Elix® electrodeionization is therefore expected to reliably deliver pure (Type 2) water that is low in PFAS.
LC-MS analysis requires ultrapure water that is reliably very low in organics and ions. These interfering trace contaminants are removed from good-quality pure water by activated carbon, photo-oxidation and ion-exchange resins. These purification technologies also help to remove PFAS even further.
When performing LC-MS or LC-MS/MS, it is also recommended to use a point-of-use polisher containing C18 reverse-phase silica. Such a polisher ensures that no traces of organics (PFAS or others) can interfere with these sensitive analyses.
Conclusion: Ultrapure water suitable for sensitive PFAS analyses
In conclusion, this study demonstrates that, even if some PFAS molecules may be present in the water feeding a water purification system, carefully selecting the system can ensure no detectable amounts of PFAS will be present in the ultrapure water produced.
An all-in-one Milli-Q® IQ 7 series system, or a combination of a good-quality pretreatment system, such as the Milli-Q® HX or IX series and a polishing system such as the Milli-Q® IQ 7000 ultrapure system, delivers ultrapure water well suited for sensitive PFAS analyses, when used with a LC-Pak® polisher.
A range of water purification solutions are available tailored to meet the needs of scientists performing PFAS testing.
Materials and Methods
Milli-Q® HX 7150 and Milli-Q® IQ 7000 water purification systems were located in Burlington, MA, USA. Tap water and ultrapure water from a Milli-Q® IQ 7000 system fitted with a LC-Pak® polisher were collected at the same time and in duplicate.
One of the challenges when testing for PFAS is that they may be present in many supplies used in the laboratory, therefore great care was taken to avoid any potential contamination of the samples with PFAS compounds. Aqueous samples never came in contact with any glass container or pipette since PFAS compounds can potentially adsorb to glass surfaces. Polypropylene copolymer containers were used for sample collection.
Water samples were analyzed by a third-party testing laboratory (Eurofins Lancaster Laboratories Environment Testing, Lancaster, PA, USA) according to the EPA 1633 draft method.6 All tests were done in duplicate.
PFAS analysis requires precision at every step. Be confident in your results with our trusted products and services for your entire PFAS workflow SigmaAldrich.com/PFAS
PFAS Testing Supplies
Acknowledgments
The authors thank Vivek Joshi, Ph.D. and Lindsay Lozeau, Ph.D., based in Burlington, MA, for their technical expertise and support.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?