1A00070
USP
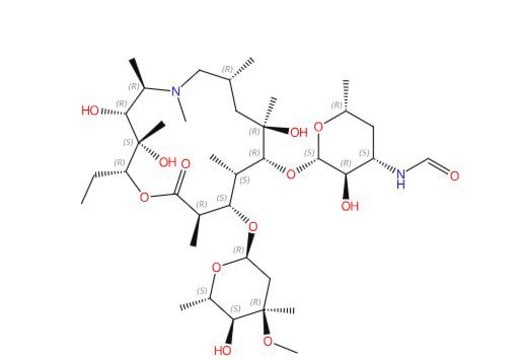
3′-(N,N-Didemethyl)Azithromycin; (Aminoazithromycin)
Pharmaceutical Analytical Impurity (PAI)
Synonyme(s) :
((2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-11-(((2S,3R,4S,6R)-4-amino-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-2- ethyl-3,4,10-trihydroxy-13-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)- 3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one, (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3-amino-3,4,6-trideoxy-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6- azacyclopentadecan-15-one)
About This Item
Produits recommandés
Qualité
pharmaceutical analytical impurity (PAI)
Agence
USP
Famille d'API
azithromycin
Fabricant/nom de marque
USP
Application(s)
pharmaceutical
Format
neat
Température de stockage
2-8°C
Description générale
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Azithromycin
Therapeutic Area: Antibiotics.
For more information about this PAI, visit here.
Application
Caractéristiques et avantages
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Remarque sur l'analyse
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique