331570
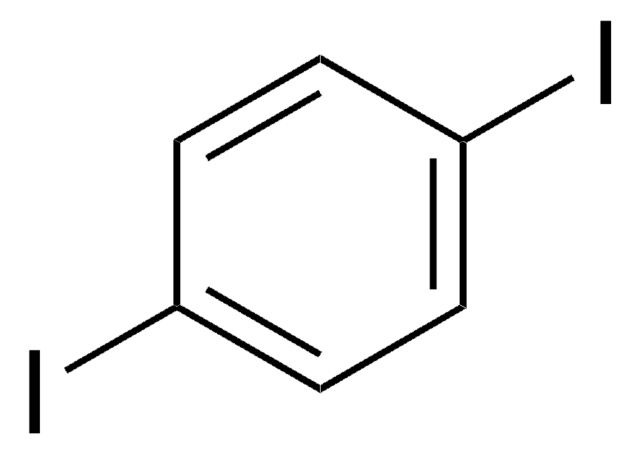
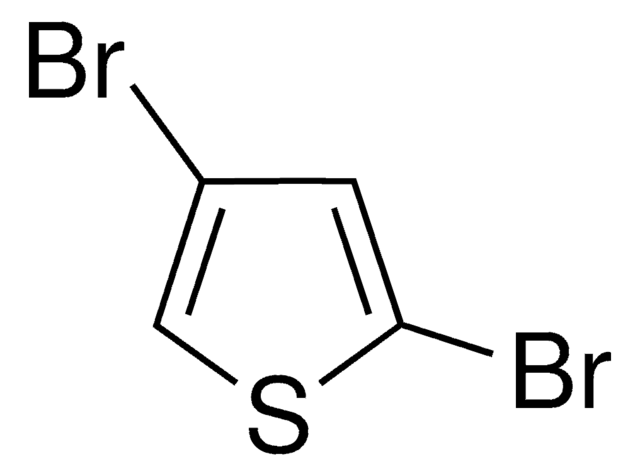
2,5-Diiodothiophene
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C4H2I2S
Numéro CAS:
Poids moléculaire :
335.93
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
98%
Forme
solid
Point d'ébullition
139-140 °C (lit.)
Pf
37-41 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
Ic1ccc(I)s1
InChI
1S/C4H2I2S/c5-3-1-2-4(6)7-3/h1-2H
Clé InChI
PNYWRAHWEIOAGK-UHFFFAOYSA-N
Description générale
The multilayer desorption behavior of 2,5-diidothiophene was studied.
Application
2,5-Diiodothiophene was used in the preparation of oligothiophene films. It was used in maskless fabrication of periodic patterns of a conjugated polymer. It was also used in fabrication of oligothiophene and polythiophene micropatterns.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
204.8 °F - closed cup
Point d'éclair (°C)
96.00 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Photochemical production of oligothiophene and polythiophene micropatterns from 2, 5-diiodothiophene on Au in UHV.
Liu G, et al.
Surface Science, 592(1), L305-L309 (2005)
Sudarshan Natarajan et al.
The journal of physical chemistry. B, 110(15), 8047-8051 (2006-04-14)
This paper describes the details of surface reactions producing >100-nm-thick conjugated polymer films. When 2,5-diiodothiophene films deposited on copper are irradiated with UV at room temperature in Ar environments, oligothiophene films are synthesized. The average conjugation length of the produced
Sudarshan Natarajan et al.
Langmuir : the ACS journal of surfaces and colloids, 21(15), 7052-7056 (2005-07-13)
Maskless fabrication of periodic patterns of a conjugated polymer is achieved by regioselective condensation of 2,5-diiodothiophene on chemically patterned substrate surfaces followed by in situ photochemical conversion of the condensed molecules into oligothiophenes and polythiophenes. This approach utilizes preferential aggregation
Guangming Liu et al.
The journal of physical chemistry. B, 110(41), 20197-20201 (2006-10-13)
The multilayer desorption behavior of 2,5-diidothiophene and the dendritic aggregation of photochemical reaction products during the desorption of 2,5-diiodothiophene multilayers have been studied. Like many other aromatic compounds, 2,5-diiodothiophene shows a multilayer desorption behavior different from the typical zeroth-order kinetics
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique