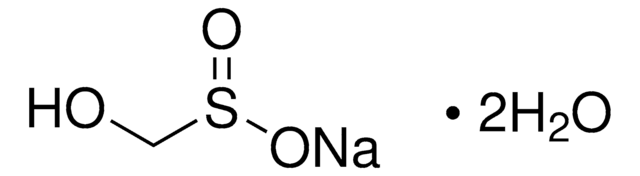All Photos(3)
About This Item
Linear Formula:
NaH2PO2 · xH2O
CAS Number:
Molecular Weight:
87.98 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.55
Recommended Products
Assay
~95% (NaH2PO2)
form
powder, crystals or chunks
SMILES string
O.[Na+].OP[O-]
InChI
1S/Na.H3O2P.H2O/c;1-3-2;/h;3H2,(H,1,2);1H2/q+1;;/p-1
InChI key
PLZNPHDJGFDNRM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Sodium hypophosphite hydrate can be used as:
It can also be used to compose the finishing solutions for the immobilization of photo-initiator (benzophenone ) onto cotton fabrics.
- A radical precursor to synthesize mono alkyl phosphinic acids via radical reaction with alkenes in the presence of Et3B as the initiator.
- A reagent for the reduction of the diazonium salt.
- A hydrogen donor in the transfer-hydrogenation reactions.
- A catalyst in the esterification reaction.
It can also be used to compose the finishing solutions for the immobilization of photo-initiator (benzophenone ) onto cotton fabrics.
Sodium hypophosphite hydrate is widely used as an esterification catalyst. It may be used to compose the finishing solutions for the incorporation of benzophenone (photoinitiator) onto cotton fabrics.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
X-Ray Photoelectron Spectroscopy of Lignocellulosic Materials Treated with Malea Ted Polypropylenes
Kazayawoko M, et al.
J. Wood Chem. Technol., 18(1), 1-26 (1998)
S Deprèle et al.
The Journal of organic chemistry, 66(20), 6745-6755 (2001-10-02)
A novel and practical approach to monosubstituted phosphinic acid (alkylphosphonous acid) derivatives from hypophosphite salts or esters is described. Phosphorus-centered radical formation is initiated with Et(3)B/O(2), and the reaction is conveniently conducted at room temperature in an open flask. In
Convenient synthesis of 4-methylhistamine and racemic. alpha., 4-dimethylhistamine and. alpha., 4-dimethylhistidine
Davey DD and Lumma Jr WC
The Journal of Organic Chemistry, 54(13), 3211-3213 (1989)
Photoactive antibacterial cotton fabrics treated by 3, 3', 4, 4'-benzophenonetetracarboxylic dianhydride.
Hong KH and Sun G.
Carbohydrate Polymers, 84(3), 1027-1032 (2011)
Effect of wood filler treatment and EBAGMA compatibilizer on morphology and mechanical properties of low density polyethylene/olive husk flour composites.
Kaci M, et al.
Express Polymer Letters, 1(7), 467-473 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service