MABS1253
Anti-O-GlcNAc Antibody, clone RL1
clone RL1, from mouse
Synonym(s):
β-GlcNAc, β-linked N-acetylglucosamine, O-GlcNAc, O-linked β-GlcNAc
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
RL1, monoclonal
species reactivity
human, Drosophila, Xenopus, rat
technique(s)
affinity binding assay: suitable
electron microscopy: suitable
immunocytochemistry: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
inhibition assay: suitable
western blot: suitable
isotype
IgMκ
shipped in
ambient
target post-translational modification
glycosylation
Gene Information
human ... OGT(8473)
General description
Specificity
Immunogen
Application
Affinity Binding Assay: A representative lot of clone RL1 & clone RL2 (Cat. No. MABS157) partially competed against each other for binding immobilized 180 kDa O-GlcNAcylated nuclear envelope protein (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
Electron Microscopy Analysis: A representative lot immunolocalized target proteins at the cytoplasmic and/or nucleoplasmic margins of the pore complex with no specific staining of the perinuclear space of isolated rat liver nuclear envelopes (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
Immunocytochemistry Analysis: Representative lots stained the nuclear envelope of paraformaldehyde-fixed, Triton X-100-permeabilized HeLa cells, NRK normal rat kidney epithelial cells and isolated rat liver nuclei by fluorescent immunocytochemistry (Yang, L., et al. (1997). J. Cell Biol. 139(5):1077-1087; Byrd, D.A., et al. (1994). J. Cell Biol. 127(6 Pt 1):1515-1526; Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
Immunofluorescence Analysis: A representative lot stained the nuclear envelope proteins in formaldehyde-fixed, Triton X-100-permeabilzied salivary glands dissected from third-instar Drosophila larvae by fluorescent immunohistochemistry (Goldberg, M., et al. (1998). Mol. Cell. Biol. 18(7):4315-4323).
Immunofluorescence Analysis: Representative lots stained the nuclear envelope of methanol-fixed xenopus ovary and rat liver cryosections by fluorescent immunohistochemistry (Featherstone, C., et al. (1988). J. Cell Biol. 107(4):1289-1297; Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
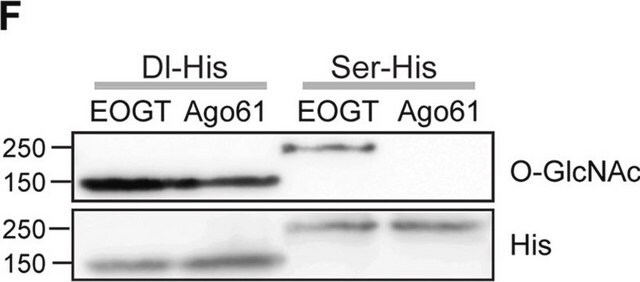
Immunoprecipitation Analysis: A representative lot immunoprecipitated several nuclear envelope proteins in salt-washed nuclear envelope preparations from rat liver and NRK normal rat kidney epithelial cells. Galactosylation of GlcNAc on nuclear envelope proteins by galactosyltransferase treatment significantly inhibited the immunoadsorption of these nuclear pore complex glycoproteins by clone RL1 (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Inhibition Analysis: A representative lot, when injected in the vegetal hemisphere of xenopus oocyte cytoplasm, inhibited nucleoplasmin nuclear import in a dose-dependent manner without affecting myoglobin nuclear import or RNA export (Featherstone, C., et al. (1988). J. Cell Biol. 107(4):1289-1297).
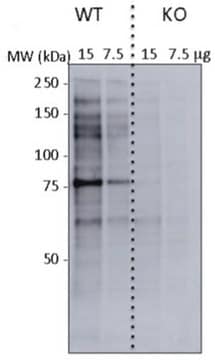
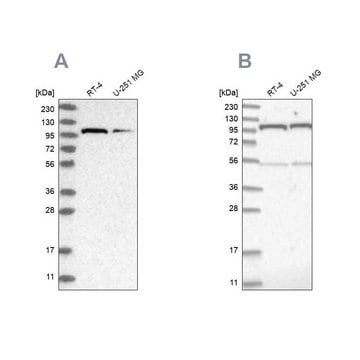
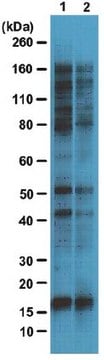
Western Blotting Analysis: Representative lots detected several nuclear envelope proteins in Xenopus oocyte nucleus extract and in salt-washed rat liver nuclear envelope preparations, including target bands of 210, 180, 145, 100, 63, 58, 54, and 45 kDa. GlcNAc removal by beta-N-acetylglucosaminidase treatment greatly reduced the detection of these nuclear pore complex glycoproteins by clone RL1 (Byrd, D.A., et al. (1994). J. Cell Biol. 127(6 Pt 1):1515-1526; Featherstone, C., et al. (1988). J. Cell Biol. 107(4):1289-1297; Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Quality
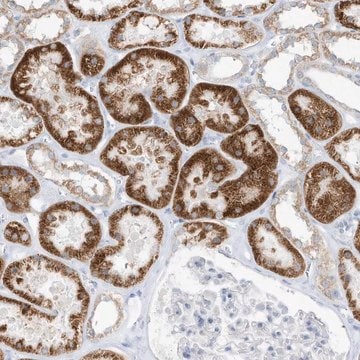
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected O-GlcNAcylated cytoplasmic and nuclear pore proteins in rat colon tissue sections.
Target description
Physical form
Storage and Stability
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze
Other Notes
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service