531464
2-Iodophenylacetonitrile
97%
Synonym(s):
2-Iodobenzyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CH2CN
CAS Number:
Molecular Weight:
243.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
refractive index
n20/D 1.618 (lit.)
bp
113-120 °C/0.5 mmHg (lit.)
density
1.75 g/mL at 25 °C (lit.)
functional group
iodo
nitrile
SMILES string
Ic1ccccc1CC#N
InChI
1S/C8H6IN/c9-8-4-2-1-3-7(8)5-6-10/h1-4H,5H2
InChI key
FPSGTRJUQLYLHE-UHFFFAOYSA-N
General description
2-Iodophenylacetonitrile is a 2-aryl substituted nitrile. It reacts with lactams to form ring-fused isoquinolinones via palladium-catalyzed carboxamidation in tandem with aldol condensation.
Application
2-Iodophenylacetonitrile may be used in the preparation of:
It may also be used in the preparation of the following nitriles:
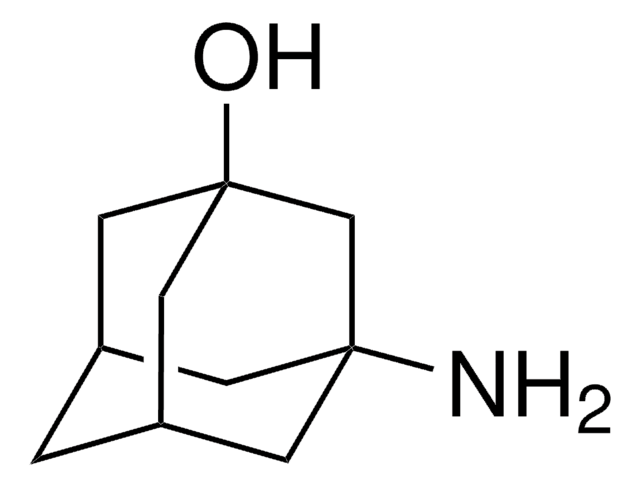
- 2?-aminobiphen-2-ylacetonitrile
- ethyl (2-iodophenyl)iminoacetate hydrochloride
- 3,4-disubstituted 2-naphthalenamines
It may also be used in the preparation of the following nitriles:
- 2-(2-iodophenyl)-2-methylpropanenitrile
- 1-(2-iodophenyl)cyclopentanecarbonitrile
- 5-bromo-2-(2-iodophenyl)pentanenitrile
- 2-(2-iodophenyl)-2-propylpentanenitrile
- 1-(2-iodophenyl)cyclohexanecarbonitrile
- 1-(2-Iodophenyl)cyclopropanecarbonitrile
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies in Acyl C? H Activation via Aryl and Alkyl to Acyl ?Through Space? Migration of Palladium.
Kesharwani T, et al.
Organic Letters, 11(12), 2591-2593 (2009)
Palladium-catalyzed borylation of ortho-substituted phenyl halides and application to the one-pot synthesis of 2,2'-disubstituted biphenyls.
Baudoin O, et al.
The Journal of Organic Chemistry, 65(26), 9268-9271 (2000)
Hirokazu Tsukamoto et al.
The Journal of organic chemistry, 81(5), 1733-1745 (2015-11-26)
1,2-Bis(diphenylphosphino)ethane (dppe)-ligated palladium(II) complexes catalyze the annulation of internal alkynes with 2-(cyanomethyl)phenylboronates to provide 3,4-disubstituted-2-naphthalenamines in good yields. The annulation reaction proceeds under mild and neutral conditions and requires methanol as an essential solvent. In addition to symmetrical alkynes, unsymmetrical
David Crich et al.
The Journal of organic chemistry, 71(9), 3452-3463 (2006-04-22)
The [1-cyano-2-(2-iodophenyl)]ethylidene group is introduced as an acetal-protecting group for carbohydrate thioglycoside donors. The group is easily introduced under mild conditions, over short reaction times, and in the presence of a wide variety of other protecting groups by the reaction
Palladium-catalyzed carboxamidation reaction and aldol condensation reaction cascade: A facile approach to ring-fused isoquinolinones.
Chouhan G and Alper H.
Organic Letters, 10(21), 4987-4990 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service