All Photos(1)
About This Item
Linear Formula:
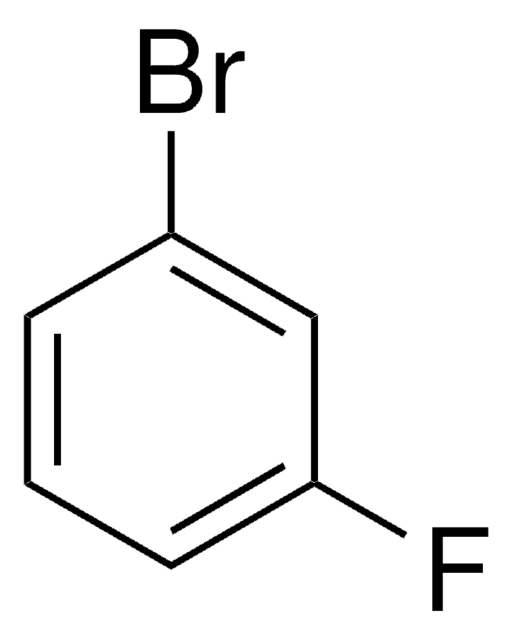
BrC6H3(F)NH2
CAS Number:
Molecular Weight:
190.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.471 (lit.)
bp
65-68 °C (lit.)
density
0.982 g/mL at 25 °C (lit.)
SMILES string
Nc1ccc(Br)c(F)c1
InChI
1S/C6H5BrFN/c7-5-2-1-4(9)3-6(5)8/h1-3H,9H2
InChI key
YTMVYYAKOPIJCZ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gang Li et al.
Chemical & pharmaceutical bulletin, 63(2), 143-146 (2014-12-11)
An improved and efficient method for the preparation of torezolid based on Suzuki cross-coupling reaction as the key step was developed on a gram scale in five steps. The total yield was 44% and the optical purity of torezolid by
Fluorinated isatins and some of their heterocyclic derivatives.
Yen VQ, et al.
The Journal of Organic Chemistry, 23(12), 1858-1861 (1958)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)