49362
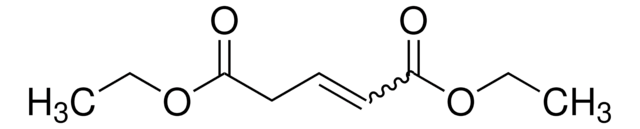
Dimethyl glutaconate
≥95.0% (GC)
Synonym(s):
Dimethyl 2-pentenoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OOCCH2CH=CHCOOCH3
CAS Number:
Molecular Weight:
158.15
Beilstein:
1724169
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (GC)
refractive index
n20/D 1.452
density
1.124 g/mL at 20 °C (lit.)
SMILES string
COC(=O)C\C=C\C(=O)OC
InChI
1S/C7H10O4/c1-10-6(8)4-3-5-7(9)11-2/h3-4H,5H2,1-2H3/b4-3+
InChI key
SKCGFFOFYXLNCG-ONEGZZNKSA-N
General description
Dimethyl glutaconate, also known as dimethyl 2-pentenoate, is a diester. It reacts with salicaldehyde in the presence of piperidine to form a coumarin-fused electron deficient diene.
Application
Dimethyl glutaconate may be used in the synthesis of:
- dimethyl 3-[(Z)-1-propenyl]glutarate
- phenanthridinone derivatives
- substituted benzenes
Components
composition: ~83% trans + ~17% cis
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A convenient method for the preparation of 3-substituted glutarate diesters.
Leotta III, et al.
The Journal of Organic Chemistry, 59(7), 1946-1946 (1994)
One-Pot Synthesis of Phenanthridinones by Using a Base-Catalyzed/Promoted Bicyclization of a, ?-Unsaturated Carbonyl Compounds with Dimethyl Glutaconate.
Li L, et al.
European Journal of Organic Chemistry, 2015(22), 4892- 4899 (2015)
Electron Deficient Dienes. 2. One Step Synthesis of a Coumarin-Fused Electron Deficient Diene and its Inverse Electron Demand Diels-Alder Reactions with Enamines.
Bodwell GJ, et al.
Synlett, 4, 477-479 (1999)
Base-Catalyzed Efficient Tandem [3+3] and [3+2+1] Annulation-Aerobic Oxidative Benzannulations.
Diallo A, et al.
Organic Letters, 14(22), 5776-5779 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service