All Photos(1)
About This Item
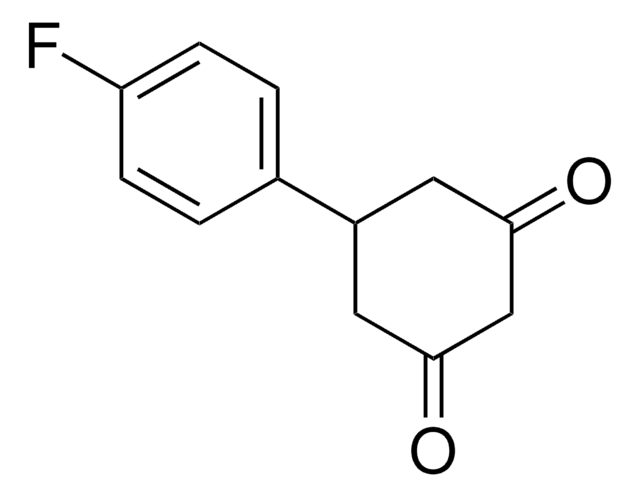
Linear Formula:
C6H5C6H7(=O)2
CAS Number:
Molecular Weight:
188.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
188 °C (lit.)
SMILES string
O=C1CC(CC(=O)C1)c2ccccc2
InChI
1S/C12H12O2/c13-11-6-10(7-12(14)8-11)9-4-2-1-3-5-9/h1-5,10H,6-8H2
InChI key
UPPYKNLSSLIIAZ-UHFFFAOYSA-N
General description
5-Phenyl-1,3-cyclohexanedione is a 1,3-diketone. Reaction of 5-phenyl-1,3-cyclohexanedione with N-substituted isatins in pyrindine has been investigated.
Application
5-Phenyl-1,3-cyclohexanedione may be used:
- in the synthesis of hexahydrobenzo[a]phenanthridin-4-one derivatives via condensation with N-arylmethylene-2-naphthylamines
- in the preparation of benzophenanthridine derivatives
- in the preparation of iodonium betaine
- in the synthesis of various 2H-pyrans by iodine-catalyzed reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of bisazomethines of the naphtalene series with 1, 3-diketones.
Kozlov SN, et al.
Russ. J. Gen. Chem., 73(9), 1424-1440 (2009)
Chunhui Dai et al.
Beilstein journal of organic chemistry, 8, 986-993 (2012-09-29)
A mild and practical synthesis of spirooxindole [1,3]oxazino derivatives from N-substituted isatins and 1,3-dicarbonyl compounds with pyridine derivatives is reported. The reactions provided good to excellent yields. Further exploration of the molecular diversity of these compounds is demonstrated through Diels-Alder
Efforts toward the synthesis of liphatic iodonium salts.
Dence JB and Roberts JD.
The Journal of Organic Chemistry, 33(3), 1251-1253 (1968)
Synthesis of Benzo [a] phenanthridine Derivatives by Condensation of N-Arylmethylene-2-naphthylamines with 5-Phenyl-and 5-(p-Methoxyphenyl)-1, 3-cyclohexanediones.
Kozlov NG, et al.
Russ. J. Gen. Chem., 72(8), 1238-1242 (2002)
Iodine-Catalyzed One-Pot Synthesis of 2H-Pyrans by Domino Knoevenagel/6p-Electrocylization.
Jung EJ, et al.
Bull. Korean Chem. Soc., 30, 2833-2836 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service