All Photos(2)
About This Item
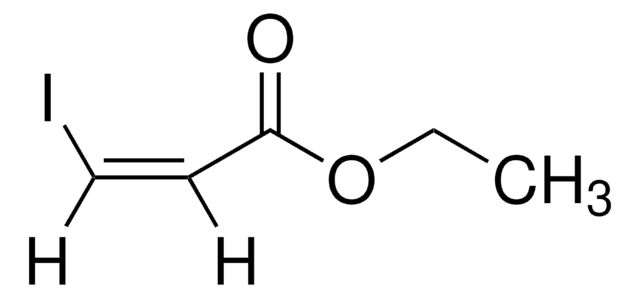
Linear Formula:
BrCH2CH2CHBrCOOCH3
CAS Number:
Molecular Weight:
259.92
Beilstein:
1757129
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (GC)
form
solid
density
1.840 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
COC(=O)C(Br)CCBr
InChI
1S/C5H8Br2O2/c1-9-5(8)4(7)2-3-6/h4H,2-3H2,1H3
InChI key
DQHIGEQXJBMKKY-UHFFFAOYSA-N
Related Categories
General description
Methyl 2,4-dibromobutyrate reacts with sodium azide in dimethylformamide to yield 2-azido-4-bromobutyrate.
Application
Methyl 2,4-dibromobutyrate was used in the preparation of stereoisomers of azetidine-2-carboxylic amide derivative.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and X-ray analysis of 1-((1S)-phenylethyl)-azetidine-(2R)-piperidinamide.
De Gelder R, et al.
Journal of Chemical Crystallography, 26(9), 639-642 (1996)
Observations on the chemistry of. alpha.-azido ester. Efficient synthesis of a potently sweet homoserine-dihydrochalcone conjugate.
DuBois GE, et al.
The Journal of Organic Chemistry, 47(7), 1319-1323 (1982)
Chuncheng Liu et al.
PloS one, 13(7), e0201551-e0201551 (2018-08-01)
MiRNAs play an important role in cell proliferation, apoptosis, and differentiation. MiR-18a is increasingly being recognized as a regulator of cancer pathogenesis. Here, we discovered that miR-18a participates in myoblasts proliferation. Expression of miR-18a was downregulated with the differentiation of
Hua Yang et al.
Cell discovery, 7(1), 90-90 (2021-10-06)
Pathogenic mycobacteria induce the formation of hypoxic granulomas during latent tuberculosis (TB) infection, in which the immune system contains, but fails to eliminate the mycobacteria. Fatty acid metabolism-related genes are relatively overrepresented in the mycobacterial genome and mycobacteria favor host-derived
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service