All Photos(1)
About This Item
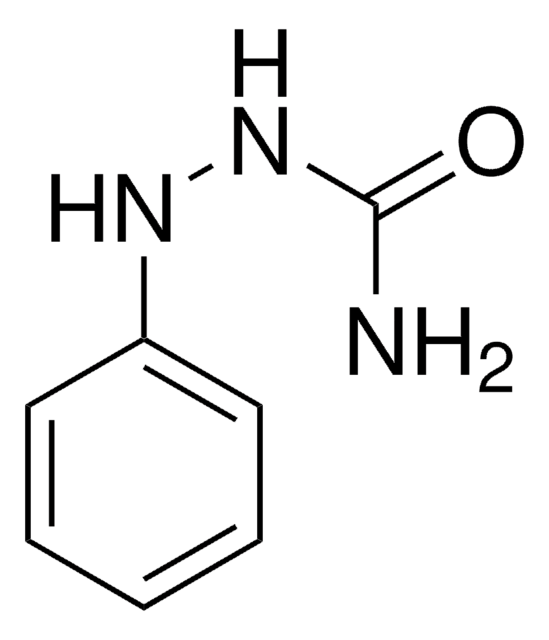
Linear Formula:
C6H5NHCONHNH2·HCl
CAS Number:
Molecular Weight:
187.63
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
215-217 °C (lit.)
solubility
alcohol: freely soluble
water: freely soluble
SMILES string
Cl.NNC(=O)Nc1ccccc1
InChI
1S/C7H9N3O.ClH/c8-10-7(11)9-6-4-2-1-3-5-6;/h1-5H,8H2,(H2,9,10,11);1H
InChI key
BNXHWDWODIHJEH-UHFFFAOYSA-N
Related Categories
Application
4-Phenylsemicarbazide hydrochloride was used in the experiment to study whether i) [U-14C]palmitate and[2-14C]palmitate are metabolized to ketone bodies and ii) free glycerol was used as an energy source by the tissue from ketotic and normal sheep.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J A Taylor et al.
The Biochemical journal, 106(1), 289-292 (1968-01-01)
Labelled ketone bodies were produced readily from [U-(14)C]palmitate, [2-(14)C]palmitate and [1-(14)C]glycerol by sheep rumen-epithelial and liver tissues in vitro. On a tissue-nitrogen basis, both tissues had similar capacities for ketogenesis. Palmitate was a ketogenic substrate in both rumen-epithelial tissue and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service