All Photos(1)
About This Item
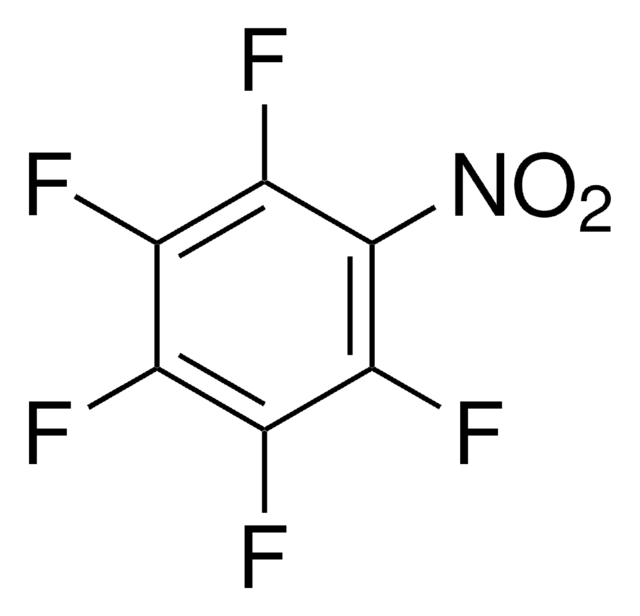
Linear Formula:
F3C6H2NH2
CAS Number:
Molecular Weight:
147.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
form
solid
bp
57 °C/22 mmHg (lit.)
mp
33-37 °C (lit.)
SMILES string
Nc1c(F)cc(F)cc1F
InChI
1S/C6H4F3N/c7-3-1-4(8)6(10)5(9)2-3/h1-2H,10H2
InChI key
BJSVKBGQDHUBHZ-UHFFFAOYSA-N
Application
2,4,6-Trifluoroaniline was used in the synthesis of:
- 3-nitro-2,4,6 -trifluoroacetanilide
- series of N′-phenyl-N-(1- phenyl cyclopentyl)-methyl ureas
- 4-substituted 2,6-difluoro N-aryl pyridinones
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T S Purchase et al.
Bioorganic & medicinal chemistry, 5(4), 739-747 (1997-04-01)
Our continued interest in developing novel, potent acyl-CoA:cholesterol acyltransferase (ACAT) inhibitors, and our discovery of several active series of disubstituted urea ACAT inhibitors, have led us to investigate a series of trisubstituted ureas that are structural hybrids of our disubstituted
Shaun R Selness et al.
Bioorganic & medicinal chemistry letters, 21(13), 4059-4065 (2011-06-07)
A series of N-aryl pyridinone inhibitors of p38 mitogen activated protein (MAP) kinase were designed and prepared based on the screening hit SC-25028 (1) and structural comparisons to VX-745 (5). The focus of the investigation targeted the dependence of potency
EPR spectroscopy of a diaza derivative of meta-xylylene.
Haider K, et al.
Tetrahedron Letters, 30(10), 1225-1228 (1989)
Mohamad Shazwan Shah Jamil et al.
Dalton transactions (Cambridge, England : 2003), 48(25), 9317-9327 (2019-06-06)
A series of imidazolium salts precursors for N-heterocyclic carbenes (NHCs) featuring fluoroaryl substituents have been prepared along with their selenides and rhodium complexes. Tests of the catalytic activity of the [Rh(cod)Cl(NHC)] complexes in the transfer hydrogenation of acetophenone with iPrOH
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service