124257
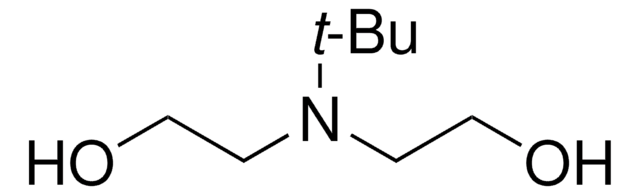
N-Butyldiethanolamine
98%
Synonym(s):
2,2′-Butyliminodiethanol, N,N-Bis(2-hydroxyethyl)butylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3N(CH2CH2OH)2
CAS Number:
Molecular Weight:
161.24
Beilstein:
1739642
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
vapor density
5.5 (vs air)
vapor pressure
1 mmHg ( 25 °C)
Assay
98%
refractive index
n20/D 1.463 (lit.)
bp
273-275 °C/741 mmHg (lit.)
mp
−70 °C (lit.)
density
0.986 g/mL at 25 °C (lit.)
SMILES string
CCCCN(CCO)CCO
InChI
1S/C8H19NO2/c1-2-3-4-9(5-7-10)6-8-11/h10-11H,2-8H2,1H3
InChI key
GVNHOISKXMSMPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Butyldiethanolamine is a tertiary amine. Densities and excess molar volumes of its binary mixtures with water have been evaluated in the temperature range of 298.15 to 353.15K.
Application
N-Butyldiethanolamine (H2bdea) may be used in the preparation of following copper(II) compounds [Hdnba = 3,5-dinitrobenzoic acid, Hpta = p-toluic acid, H2tpa = terephthalic acid):
- mononuclear [Cu(Hbdea)2]·2Hdnba
- dinuclear [Cu2(μ-Hbdea)2(N3)2]
- [Cu2(μ-Hbdea)2(pta)2]·2H2O
- 1D polymeric [Cu2(μ-Hbdea)2(μ-tpa)]n.2nH2O
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
265.1 °F - closed cup
Flash Point(C)
129.5 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Densities and volumetric properties of the aqueous solutions of 2-amino-2-methyl-1-propanol, n-butyldiethanolamine and n-propylethanolamine at temperatures from 298.15 to 353.15 K.
Chan C, et al.
Fluid Phase Equilibria, 198(2), 239-250 (2002)
Katrin R Gruenwald et al.
Dalton transactions (Cambridge, England : 2003), (12)(12), 2109-2120 (2009-03-11)
The new mononuclear [Cu(Hbdea)(2)].2Hdnba (), dinuclear [Cu(2)(mu-Hbdea)(2)(N(3))(2)] () and [Cu(2)(mu-Hbdea)(2)(pta)(2)].2H(2)O (), and 1D polymeric [Cu(2)(mu-Hbdea)(2)(mu-tpa)](n).2nH(2)O () copper(II) compounds have been prepared by self-assembly, in aqueous alkali medium and at ambient conditions, from Cu(II) acetate, N-butyldiethanolamine (H(2)bdea) and the corresponding auxiliary
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service