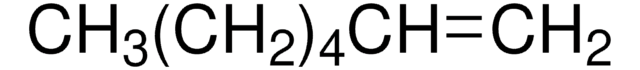125431
Cyclohexene
inhibitor-free, ReagentPlus®, 99%
Synonym(s):
Tetrahydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C6H10
CAS Number:
Molecular Weight:
82.14
Beilstein:
906737
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
2.8 (vs air)
Quality Level
vapor pressure
160 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
autoignition temp.
590 °F
expl. lim.
5 %
refractive index
n20/D 1.446 (lit.)
bp
83 °C (lit.)
mp
−104 °C (lit.)
solubility
water: insoluble
density
0.811 g/mL at 25 °C (lit.)
SMILES string
C1CCC=CC1
InChI
1S/C6H10/c1-2-4-6-5-3-1/h1-2H,3-6H2
InChI key
HGCIXCUEYOPUTN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cyclohexene is a cyclic alkene. Kinetics of its reaction with hydrogen in gas and liquid phases over different palladium catalysts supported on silica gel and alumina (temperature range = 264 to 308K and hydrogen pressures = 4 to 89kPa) was investigated. Cyclohexene is formed during the batch-wise partial hydrogenation of benzene over ruthenium catalysts in the presence of an aqueous zinc sulphate solution. Cyclohexene undergoes enantioselective catalytic oxidation in the presence of hydrogen peroxide over environment-friendly peroxytungstate-organic complex catalysts to afford adipic acid. Selective hydrogenation of benzene has been reported to afford cyclohexene. It is an important precursor for the preparation of various chemicals such as cyclohexanol, cyclohexene hydroperoxide, etc.
Application
Cyclohexene has been used to investigate the kinetics of its liquid-phase hydrogenation reaction in the presence of Pd-Al catalyst supported on biomorphic carbon. It may be used for the industrial preparation of cyclohexanol.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
1.4 °F
Flash Point(C)
-17 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalytic hydrogenation of cyclohexene: 3. Gas-phase and liquid-phase reaction on supported palladium.
Gonzo EE and Boudart M.
J. Catal., 52(3), 462-471 (1978)
Kinetics of liquid phase cyclohexene hydrogenation on Pd-Al/biomorphic carbon catalysts.
Caza?a F, et al.
Catalysis Today, 14-14 (2014)
Yuan Fang et al.
The journal of physical chemistry letters, 10(3), 468-473 (2019-01-03)
The adsorption of limonene, a common organic compound found in indoor air, on hydrophilic surfaces such as glass (SiO2), a prevalent surface in the indoor environment, is poorly understood. In this study, we have investigated the interaction of limonene and
Oxidative decarboxylation of benzilic acid by a biomimetic iron(II) complex: evidence for an iron(IV)-oxo-hydroxo oxidant from O2.
Sayantan Paria et al.
Angewandte Chemie (International ed. in English), 50(47), 11129-11132 (2011-10-01)
Sylvain Dalençon et al.
The Journal of organic chemistry, 76(19), 8059-8063 (2011-08-26)
The asymmetric synthesis of novel cyclohexene nucleoside analogues 12 and 15 is described. An enantiospecific Diels-Alder reaction between (E,E)-diene 2 and (+)-5-(d-mentyloxy)-2(5H)-furanone 3 provided the cycloadduct isomer 4. Three additional steps yielded amine 8 allowing the constructions of the thymine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service