W237809
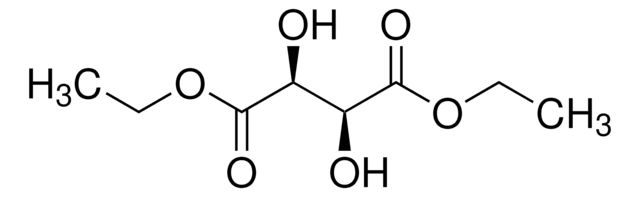
Diethyl L-tartrate
≥99%, FG
Synonym(s):
(+)-Diethyl L-tartrate, L-(+)-Tartaric acid diethyl ester
About This Item
Fragrance grade
Kosher
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥99%
optical activity
[α]20/D +8.5°, neat
refractive index
n20/D 1.446 (lit.)
bp
280 °C (lit.)
density
1.204 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
fruity; wine-like
SMILES string
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
InChI key
YSAVZVORKRDODB-PHDIDXHHSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service