247731
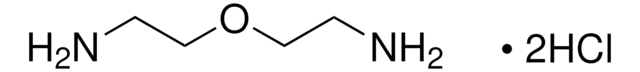
Hexamethylenediamine dihydrochloride
99%
Synonym(s):
1,6-Hexanediamine dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2N(CH2)6NH2 · 2HCl
CAS Number:
Molecular Weight:
189.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
256-257 °C (lit.)
solubility
water: freely soluble
SMILES string
Cl.Cl.NCCCCCCN
InChI
1S/C6H16N2.2ClH/c7-5-3-1-2-4-6-8;;/h1-8H2;2*1H
InChI key
XMVQMBLTFKAIOX-UHFFFAOYSA-N
General description
Toxicity of hexamethylenediamine dihydrochloride has been investigated. Hexamethylenediamine dihydrochloride is also known as 1,6-diaminohexane dihydrochloride, 1,6-hexamethylenediamine dihydrochloride, 1,6- hexylenediamine dihydrochloride or 1,6-diamino-n-hexane dihydrochloride. Hexamethylenediamine dihydrochloride on fusion of 1:6-di-(N3-cyano-N1-guanidino)-hexane yields polymeric diguanides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
850. Bisdiguanides having antibacterial activity.
Rose FL andSwain G.
Journal of the Chemical Society, 4422-4425 (1956)
Charles Hebert
Toxicity report series, 24, 1-D8-1-D8 (1993-03-01)
1,6-Hexanediamine (HDA) is an aliphatic amine that is produced in large volumes in the United States. HDA is widely used as a corrosion inhibitor in lubricants and as an intermediate in the industrial synthesis of paints, resins, inks, and textiles.
Bo Tao et al.
Polymers, 12(5) (2020-05-20)
Dopamine-modified hyaluronic acid (HA-DOP) was chosen as the drug carrier in this study, and Cu2+ was selected from among Cu2+, Zn2+, Fe2+, and Ca2+ as the central atom. 6-Mercaptopurine (6-MP) was conjugated with HA through a coordination reaction. HA-DOP-copper-MP (HA-DOP-Cu-MP)
Xiang Mei Yan et al.
Journal of biomaterials applications, 27(2), 179-186 (2011-04-30)
HA-HMDA hydrogels were developed by direct amide bond formation between the carboxyl groups of hyaluronic acid (HA) and hexamethylenediamine (HMDA) with an optimized carboxyl group modification in the preliminary experiment. However, these HA-HMDA hydrogels transformed into an unstable liquid form
Linda G T Gaines et al.
The Annals of occupational hygiene, 54(6), 678-691 (2010-06-10)
Urinary 1,6-hexamethylene diamine (HDA) may serve as a biomarker for systemic exposure to 1,6-hexamethylene diisocyanate (HDI) in occupationally exposed populations. However, the quantitative relationships between dermal and inhalation exposure to HDI and urine HDA levels have not been established. We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service