All Photos(1)
About This Item
Empirical Formula (Hill Notation):
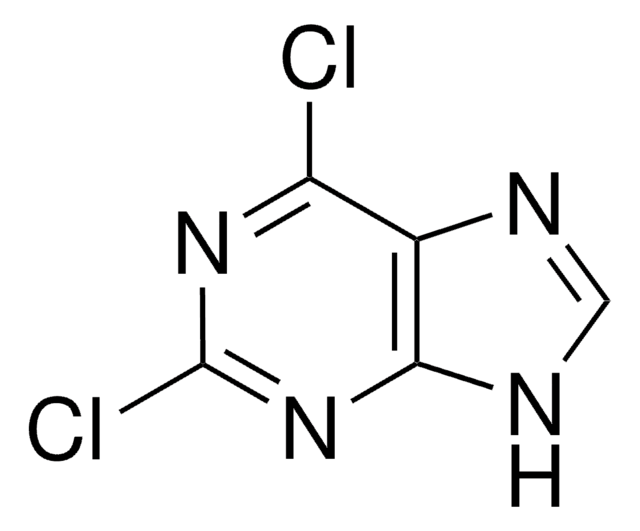
C5H3ClN4
CAS Number:
Molecular Weight:
154.56
Beilstein:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
mp
>300 °C (dec.) (lit.)
solubility
DMF: soluble 5%, clear, colorless to yellow
functional group
chloro
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The acid-catalyzed reaction of 6-chloropurine with 3,4-di-O-acetyl-D-xylal has been investigated.
Application
6-Chloropurine has been used in the preparation of 9-alkylpurines via alkylation with various substituted alkyl halides in DMSO. It was also used in the preparation of 6-succinoaminopurine.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 6-succinoaminopurine.
C E CARTER
The Journal of biological chemistry, 223(1), 139-146 (1956-11-01)
Synthesis of Potential Anticancer Agents. XXVI. The Alkylation of 6-Chloropurine2.
Montgomery JA and Temple Jr C.
Journal of the American Chemical Society, 83(3), 630-635 (1961)
Heterocyclic N-glycosides-V: Synthesis of unsaturated N-glycosides from 6-chloropurine and derivatives of d-xylal and l-arabinal. A conformational NMR study.
Fuertes M, et al.
Tetrahedron, 26(20), 4823-4837 (1970)
V Gurvich et al.
Nucleosides & nucleotides, 18(10), 2327-2333 (2000-01-05)
Tetrabutylammonium triphenydifluorosilicate (TBAT) has been found to be a useful reagent for the conversion of 6-chloropurine nucleosides to 6-fluoropurine derivatives. The 6-chloropurine nucleosides were reacted with trimethylamine to form quaternary trimethylammonium salts which were treated in situ with TBAT in
Masahiro Ikejiri et al.
Nucleic acids symposium series (2004), (51)(51), 439-440 (2007-11-22)
A series of nucleoside analogues whose 5'-hydroxyl groups are masked by various protective groups were synthesized and evaluated to develop novel anti- hepatitis C virus (HCV) agents. Among the several analogues that showed anti-HCV potency, a 5'-O-benzoyl-2'-deoxyribonucleoside analogue exhibited high
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service