ALD00564
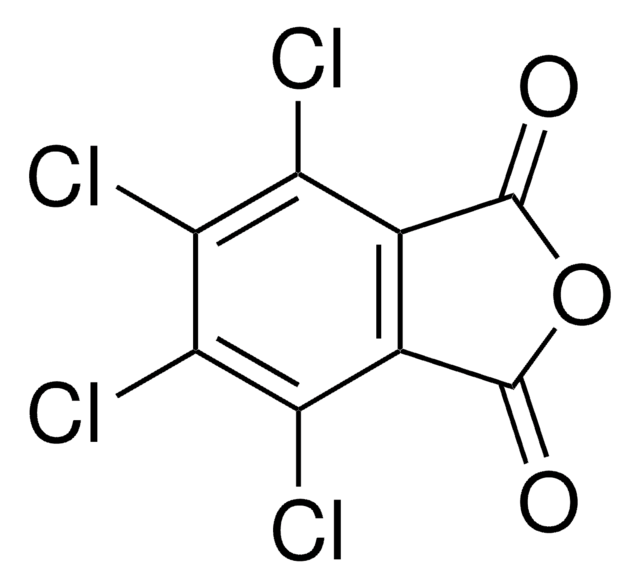
N-Hydroxytetrachlorophthalimide
Synonym(s):
4,5,6,7-Tetrachloro-2-hydroxy-1H-isoindole-1,3(2H)-dione, Tetrachloro-N-hydroxyphthalimide
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: oxidant
SMILES string
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI key
UTRBHXSKVVPTLY-UHFFFAOYSA-N
General description
Application
Other Notes
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Related Content
The Baran Group works with Sigma-Aldrich in providing a portfolio of zinc-based reagents promoting difluoromethylation, trifluoromethylation, trifluoroethylation and isopropylation of aryl and heteroaryl motifs. Baran’s lab has also helped introduce a portable desaturase (Tz0Cl), which promotes the installation of alcohol and amine groups and leaves behind a highly useful tosyl group for further transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service