All Photos(1)
About This Item
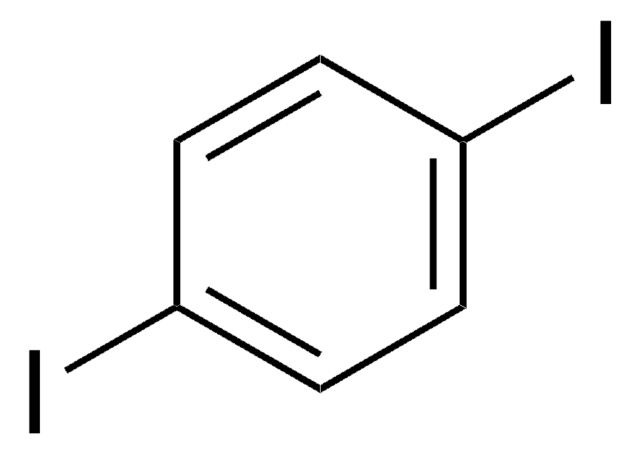
Empirical Formula (Hill Notation):
C6H4I2
CAS Number:
Molecular Weight:
329.90
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
storage condition
protect from light
mp
34-37 °C (lit.)
storage temp.
2-8°C
SMILES string
Ic1cccc(I)c1
InChI
1S/C6H4I2/c7-5-2-1-3-6(8)4-5/h1-4H
InChI key
SFPQFQUXAJOWNF-UHFFFAOYSA-N
General description
1,3-Diiodobenzene is a halogenated benzene derivative. Its reaction with phenylboronic acid in the presence of CuI, DABCO (1,4-diazabicyclo[2.2.2]octane) and TBAB (n-Bu4NBr) has been analyzed. 1,3-Diiodobenzene undergoes coupling with 2-methylthiophene in the presence of Ir/Ag2CO3 to afford meta-linked isomer of thiophene-benzene-thiophene triad.
Application
1,3-Diiodobenzene may be used in the synthesis of:
- 3,5-bis(perfluorodecyl)phenylboronic acid
- epitaxially aligned and separated polyphenylene lines on Cu(110)
- 1,3-bis(4-ethynyl-2,5-dibutoxyphenyl-1-ethynyl)benzene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3, 5-Bis (perfluorodecyl) phenylboronic acid as an easily recyclable direct amide condensation catalyst.
Ishihara K, et al.
Synlett, 2001(09), 1371-1374 (2001)
Synthesis, Chain Rigidity, and Luminescent Properties of Poly [(1, 3-phenyleneethynylene)-a lt-tris (2, 5-dialkoxy-1, 4-phenyleneethynylene)] s.
Chu Q, et al.
Macromolecules, 35(20), 7569-7574 (2002)
Jin-Heng Li et al.
The Journal of organic chemistry, 72(6), 2053-2057 (2007-02-09)
In the presence of TBAB, CuI-catalyzed Suzuki-Miyaura cross-coupling of vinyl halides and aryl halides with arylboronic acids was conducted smoothly to afford the corresponding diarylethenes and polyaryls in moderate to good yields using DABCO (1,4-diazabicyclo[2.2.2]octane) as the ligand. We also
Benoît Join et al.
Angewandte Chemie (International ed. in English), 48(20), 3644-3647 (2009-04-09)
Efficient couplings using equimolar quantities of each coupling partner and multiple C-H bond arylation reactions are achieved with an Ir-based catalytic system for the C-H bond arylation of electron-rich heteroarenes with iodoarenes to construct extended pi-systems. The dramatic ligand effect
J A Lipton-Duffin et al.
Small (Weinheim an der Bergstrasse, Germany), 5(5), 592-597 (2009-02-26)
The surface-mediated synthesis of epitaxially aligned and separated polyphenylene lines on Cu(110) by exploiting the Ullmann dehalogenation reaction is reported. Scanning tunneling microscopy (STM) and X-ray photoelectron spectroscopy (XPS) show that the C-I bonds of 1,4-diiodobenzene and 1,3-diiodobenzene (C(6)H(4)I(2)) are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service